Magnesium Iodide Chemical Equation . M g2+ +2i − → m gi 2. The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). Gas phase ion energetics data. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. The magnesium iodide formula is mgi2. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. Magnesium metal forms a doubly charged cation. Diiodomagnesium is a crystalline solid white in. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. In this video we'll write the correct formula for magnesium iodide (mgi2). Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Iodide forms a singly charged anion.
from www.numerade.com
The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). The magnesium iodide formula is mgi2. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. M g2+ +2i − → m gi 2. It forms various hydrates mgi2·xh2o. Iodide forms a singly charged anion. Magnesium metal forms a doubly charged cation. Diiodomagnesium is a crystalline solid white in. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Gas phase ion energetics data.
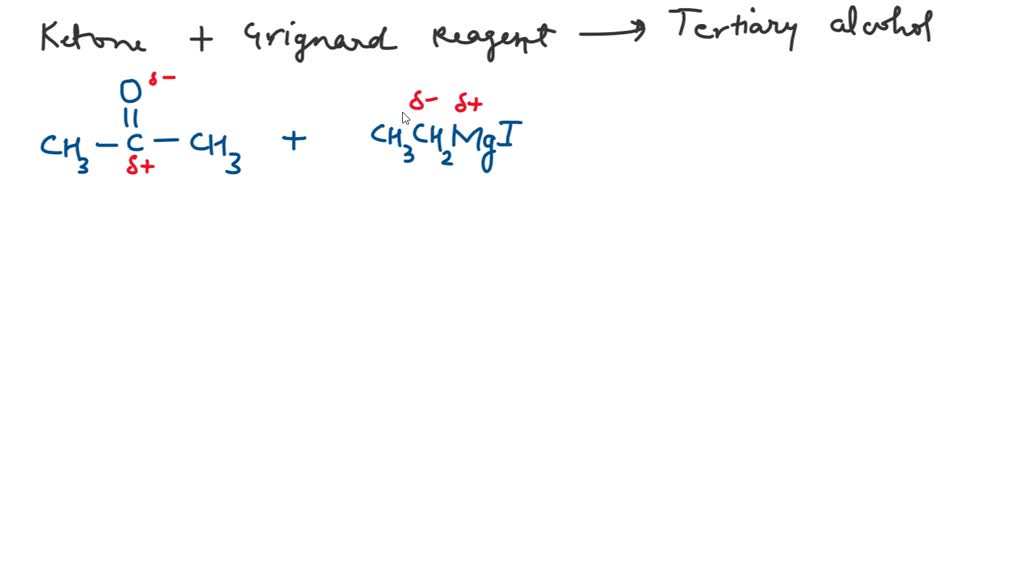
SOLVED Which of the following compounds on reaction with ethyl
Magnesium Iodide Chemical Equation It forms various hydrates mgi2·xh2o. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Gas phase ion energetics data. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. Iodide forms a singly charged anion. Diiodomagnesium is a crystalline solid white in. It forms various hydrates mgi2·xh2o. Magnesium metal forms a doubly charged cation. The magnesium iodide formula is mgi2. M g2+ +2i − → m gi 2. The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). In this video we'll write the correct formula for magnesium iodide (mgi2).
From kunduz.com
[ANSWERED] A 1 628g sample of a hydrate of Magnesium iodide Mgl xH2O Magnesium Iodide Chemical Equation In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. In this video we'll write the correct formula for magnesium iodide (mgi2). The magnesium iodide formula is mgi2. Iodide forms a singly charged anion. Magnesium metal forms a doubly charged cation. M g2+ +2i − → m. Magnesium Iodide Chemical Equation.
From www.doubtnut.com
To prepare butan2ol from methyl magnesium iodide. The compound requi Magnesium Iodide Chemical Equation Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. M g2+ +2i − → m gi 2. It forms various hydrates mgi2·xh2o. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. The magnesium iodide formula is mgi2. Magnesium metal forms a doubly charged cation. Gas phase ion energetics. Magnesium Iodide Chemical Equation.
From www.youtube.com
How to Write the Formula for MgO (Magnesium oxide) YouTube Magnesium Iodide Chemical Equation Gas phase ion energetics data. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. In this video we'll write the correct formula for magnesium iodide (mgi2). Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. It forms various hydrates mgi2·xh2o. The. Magnesium Iodide Chemical Equation.
From imgbin.com
Copper(I) Iodide Magnesium Iodide Crystal Structure Molecule PNG Magnesium Iodide Chemical Equation The magnesium iodide formula is mgi2. The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). Diiodomagnesium is a crystalline solid white in. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. Gas phase ion energetics data. Magnesium metal forms a doubly charged cation. M g2+ +2i. Magnesium Iodide Chemical Equation.
From molekula.com
Purchase Magnesium iodide anhydrous [10377589] online • Catalog Magnesium Iodide Chemical Equation It forms various hydrates mgi2·xh2o. Gas phase ion energetics data. The magnesium iodide formula is mgi2. Magnesium metal forms a doubly charged cation. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). Magnesium iodide is a highly stable compound under normal conditions, but it. Magnesium Iodide Chemical Equation.
From stock.adobe.com
Magnesium iodide mgi2 molecule. Simple molecular formula consisting of Magnesium Iodide Chemical Equation Gas phase ion energetics data. Diiodomagnesium is a crystalline solid white in. The magnesium iodide formula is mgi2. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. In this. Magnesium Iodide Chemical Equation.
From www.techscience.com
CMES Free FullText Algebraic Properties for Molecular Structure of Magnesium Iodide Chemical Equation Diiodomagnesium is a crystalline solid white in. The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). Magnesium metal forms a doubly charged cation. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. M g2+ +2i − → m gi 2. Iodide forms a singly charged anion. In the magnesium. Magnesium Iodide Chemical Equation.
From www.pw.live
Magnesium Iodide Formula, Structure And Properties Magnesium Iodide Chemical Equation Diiodomagnesium is a crystalline solid white in. The magnesium iodide formula is mgi2. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Magnesium metal forms a doubly charged cation. M g2+ +2i − → m gi 2. Iodide forms a singly charged anion. Magnesium iodide is a highly stable compound under normal. Magnesium Iodide Chemical Equation.
From favpng.com
Magnesium Iodide Chemical Compound Hydrate, PNG, 1033x1100px, Magnesium Magnesium Iodide Chemical Equation In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms.. Magnesium Iodide Chemical Equation.
From www.chegg.com
Solved What is the formula weight of magnesium iodide? How Magnesium Iodide Chemical Equation The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). Iodide forms a singly charged anion. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. In this video we'll write the correct formula for magnesium iodide (mgi2). M g2+ +2i − → m gi. Magnesium Iodide Chemical Equation.
From www.numerade.com
SOLVED 'Magnesium reacts with iodine gas at high temperatures to form Magnesium Iodide Chemical Equation The magnesium iodide formula is mgi2. In this video we'll write the correct formula for magnesium iodide (mgi2). In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. The molecular or chemical formula of. Magnesium Iodide Chemical Equation.
From www.shutterstock.com
Magnesium Iodide Formula Handwritten Chemical Formula Stock Magnesium Iodide Chemical Equation In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. In this video we'll write the correct formula for magnesium iodide (mgi2). M g2+ +2i − →. Magnesium Iodide Chemical Equation.
From www.youtube.com
Draw the Lewis Structure of MgI2 (magnesium iodide) YouTube Magnesium Iodide Chemical Equation It forms various hydrates mgi2·xh2o. M g2+ +2i − → m gi 2. The magnesium iodide formula is mgi2. Gas phase ion energetics data. Iodide forms a singly charged anion. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains. Magnesium Iodide Chemical Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + AgNO3 = Ag + Mg(NO3)2 Magnesium Iodide Chemical Equation Iodide forms a singly charged anion. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Gas phase ion energetics data. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. Magnesium Iodide Chemical Equation.
From klaidjyaa.blob.core.windows.net
Magnesium Iron Silicate Hydroxide Formula at Rodney Pendergast blog Magnesium Iodide Chemical Equation Iodide forms a singly charged anion. The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). In this video we'll write the correct formula for magnesium iodide (mgi2). It forms various hydrates mgi2·xh2o. The magnesium iodide formula is mgi2. Gas phase ion energetics data. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon. Magnesium Iodide Chemical Equation.
From www.numerade.com
SOLVED Which of the following compounds on reaction with ethyl Magnesium Iodide Chemical Equation The magnesium iodide formula is mgi2. M g2+ +2i − → m gi 2. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. In this video. Magnesium Iodide Chemical Equation.
From www.bartleby.com
Answered The compound magnesium iodide, MgI2 is… bartleby Magnesium Iodide Chemical Equation Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. It forms various hydrates mgi2·xh2o. M g2+ +2i − → m gi 2. The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). The magnesium iodide formula is mgi2. Magnesium iodide is an inorganic compound with the chemical. Magnesium Iodide Chemical Equation.
From www.numerade.com
SOLVED Draw the Lewis Dot Diagram for Mg (magnesium) (iodine) (1 Magnesium Iodide Chemical Equation Gas phase ion energetics data. The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). Diiodomagnesium is a crystalline solid white in. M g2+ +2i − → m gi 2. The magnesium iodide formula is mgi2. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. Magnesium metal. Magnesium Iodide Chemical Equation.
From brainly.com
The diagram shows how magnesium and iodine atoms form magnesium iodide Magnesium Iodide Chemical Equation Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Iodide forms a singly charged anion. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. Diiodomagnesium is a crystalline solid white in. In this video we'll write the correct formula for magnesium. Magnesium Iodide Chemical Equation.
From www.numerade.com
SOLVED Text Name of Compound Chemical Formula Type of compound ionic Magnesium Iodide Chemical Equation Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. It forms various hydrates mgi2·xh2o. Gas phase ion energetics data. Magnesium metal forms a doubly charged cation. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Diiodomagnesium is a crystalline solid white in. The molecular or. Magnesium Iodide Chemical Equation.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Magnesium Iodide Chemical Equation In this video we'll write the correct formula for magnesium iodide (mgi2). Diiodomagnesium is a crystalline solid white in. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Gas phase ion energetics data. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. The magnesium iodide formula is mgi2.. Magnesium Iodide Chemical Equation.
From www.youtube.com
Equation for Mg(NO3)2 + H2O (Magnesium nitrate + Water) YouTube Magnesium Iodide Chemical Equation Gas phase ion energetics data. Magnesium metal forms a doubly charged cation. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Diiodomagnesium is a crystalline solid white in. The magnesium iodide formula is mgi2. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one. Magnesium Iodide Chemical Equation.
From www.shutterstock.com
Magnesium Iodide Molecular Structure Formula Periodic Stock Vector Magnesium Iodide Chemical Equation Gas phase ion energetics data. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. It forms various hydrates mgi2·xh2o. In this video we'll write the correct formula for magnesium iodide (mgi2). The molecular. Magnesium Iodide Chemical Equation.
From www.youtube.com
How to Balance Mg + I2 = MgI2 (Magnesium + Iodine gas) YouTube Magnesium Iodide Chemical Equation Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Iodide forms a singly charged anion. Diiodomagnesium is a crystalline solid white in. M g2+ +2i − → m gi 2. Gas phase ion energetics data. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form. Magnesium Iodide Chemical Equation.
From www.pw.live
Magnesium Iodide Formula, Structure And Properties Magnesium Iodide Chemical Equation The magnesium iodide formula is mgi2. Magnesium iodide is an inorganic compound with the chemical formula mg i 2. M g2+ +2i − → m gi 2. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. It forms various hydrates mgi2·xh2o. Magnesium iodide is a highly stable compound under normal conditions, but. Magnesium Iodide Chemical Equation.
From www.youtube.com
How to Write the Formula for Magnesium iodide YouTube Magnesium Iodide Chemical Equation In this video we'll write the correct formula for magnesium iodide (mgi2). The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). Diiodomagnesium is a crystalline solid white in. M g2+ +2i − → m gi 2. Magnesium metal forms a doubly charged cation. Gas phase ion energetics data. The magnesium iodide formula is mgi2. It forms various. Magnesium Iodide Chemical Equation.
From www.slideserve.com
PPT Nomenclature Chemical Formulas Reactions PowerPoint Presentation Magnesium Iodide Chemical Equation Gas phase ion energetics data. Diiodomagnesium is a crystalline solid white in. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). In this video we'll write the correct formula for magnesium iodide (mgi2). It forms various hydrates. Magnesium Iodide Chemical Equation.
From www.chegg.com
Solved Magnesium Reacts With Iodine Gas At High Temperatu... Magnesium Iodide Chemical Equation The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). In this video we'll write the correct formula for magnesium iodide (mgi2). Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Gas phase ion energetics data. M g2+ +2i − → m gi 2. Iodide forms a singly charged anion. Magnesium metal forms a. Magnesium Iodide Chemical Equation.
From www.alamy.com
MgI2 Magnesium Iodide. Chemical compound. CAS number 10377589 Stock Magnesium Iodide Chemical Equation Magnesium metal forms a doubly charged cation. It forms various hydrates mgi2·xh2o. The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide. Magnesium Iodide Chemical Equation.
From www.guidechem.com
Magnesium iodide ethyn1ide (1/1/1) 121526781 wiki Magnesium Iodide Chemical Equation The magnesium iodide formula is mgi2. In this video we'll write the correct formula for magnesium iodide (mgi2). Gas phase ion energetics data. In the magnesium iodide compound, magnesium loses two electrons to become a mg²⁺ ion, while iodine gains one electron to form i⁻ ions. M g2+ +2i − → m gi 2. Magnesium metal forms a doubly charged. Magnesium Iodide Chemical Equation.
From www.youtube.com
Equation for MgI2 + H2O (Magnesium iodide + Water) YouTube Magnesium Iodide Chemical Equation Diiodomagnesium is a crystalline solid white in. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce magnesium oxide and. Iodide forms a singly charged anion. The molecular or chemical formula of magnesium iodide is mgi. Magnesium Iodide Chemical Equation.
From stock.adobe.com
Magnesium Iodide MgI2 molecule. Simple molecular formula consisting of Magnesium Iodide Chemical Equation The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). Magnesium iodide is an inorganic compound with the chemical formula mg i 2. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. The magnesium iodide formula is mgi2. Iodide forms a singly charged anion. Gas phase ion energetics data. Diiodomagnesium. Magnesium Iodide Chemical Equation.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Magnesium Iodide Chemical Equation Diiodomagnesium is a crystalline solid white in. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. Magnesium metal forms a doubly charged cation. Gas phase ion energetics data. Iodide forms a singly charged anion. M g2+ +2i − → m gi 2. The magnesium iodide formula is mgi2. It forms various hydrates. Magnesium Iodide Chemical Equation.
From www.doubtnut.com
Methyl magnesium iodide is converted to ethyl magnesium iodide Magnesium Iodide Chemical Equation The molecular or chemical formula of magnesium iodide is mgi 2 (anhydrous). Diiodomagnesium is a crystalline solid white in. It forms various hydrates mgi2·xh2o. Gas phase ion energetics data. Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. M g2+ +2i − → m gi 2. Magnesium metal forms a doubly charged. Magnesium Iodide Chemical Equation.
From guides.byjusweb.com
Magnesium Iodide Formula Definition, Concepts and Examples Magnesium Iodide Chemical Equation Magnesium iodide is an ionic compound formed by the combination of magnesium (mg) and iodine (i) atoms. In this video we'll write the correct formula for magnesium iodide (mgi2). Magnesium metal forms a doubly charged cation. Diiodomagnesium is a crystalline solid white in. Magnesium iodide is a highly stable compound under normal conditions, but it decomposes upon heating to produce. Magnesium Iodide Chemical Equation.