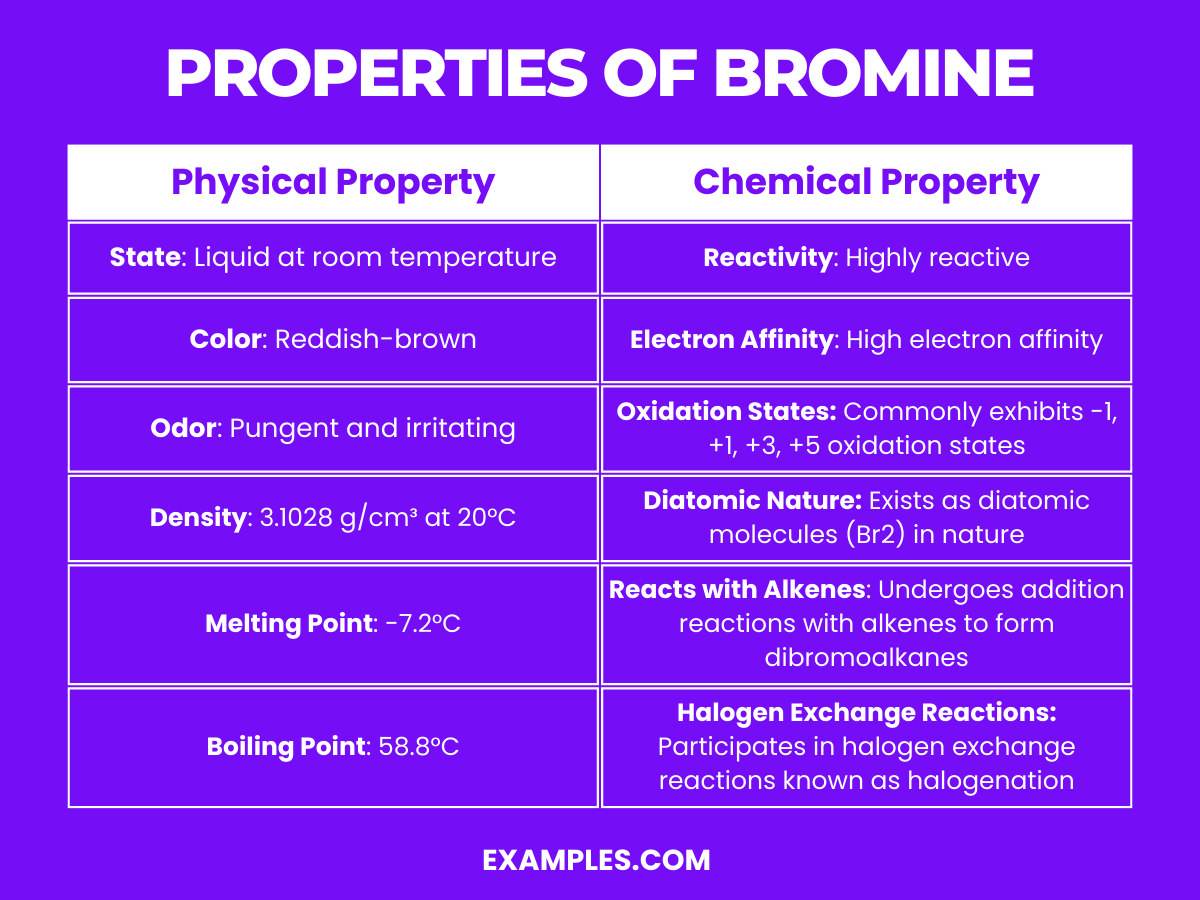
Bromine Chloride Uses . Substitution reactions that use brcl are generally much faster than those that use bromine alone. Risk assessment, environmental, and health hazard. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Sushma, in hazardous gases, 2021. Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine(i) chloride is a chloride of bromine. Preformed bromine chloride can also be used for this purpose. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals.
from www.examples.com
Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Bromine(i) chloride is a chloride of bromine. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. Sushma, in hazardous gases, 2021. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Preformed bromine chloride can also be used for this purpose. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Risk assessment, environmental, and health hazard.
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds
Bromine Chloride Uses Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. Risk assessment, environmental, and health hazard. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Preformed bromine chloride can also be used for this purpose. Bromine(i) chloride is a chloride of bromine. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Sushma, in hazardous gases, 2021. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals.
From www.numerade.com
SOLVED For the following reaction, 6.37 grams of chlorine gas are Bromine Chloride Uses Substitution reactions that use brcl are generally much faster than those that use bromine alone. Risk assessment, environmental, and health hazard. Bromine(i) chloride is a chloride of bromine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Sushma, in hazardous gases, 2021. Preformed bromine chloride can also be used for this. Bromine Chloride Uses.
From www.grainger.com
BRADY Chemical Sign Bromine Potential Hazards, Fiberglass, 10 in Ht, 7 Bromine Chloride Uses It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Sushma, in hazardous gases, 2021. Preformed bromine chloride can also be used for this purpose. Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds. Bromine Chloride Uses.
From www.numerade.com
SOLVED Chlorine can replace bromine in bromide compounds, forming Bromine Chloride Uses Risk assessment, environmental, and health hazard. Preformed bromine chloride can also be used for this purpose. Bromine(i) chloride is a chloride of bromine. Sushma, in hazardous gases, 2021. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride,. Bromine Chloride Uses.
From www.bigstockphoto.com
Bromine Monochloride Image & Photo (Free Trial) Bigstock Bromine Chloride Uses Bromine(i) chloride is a chloride of bromine. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Risk assessment, environmental, and health hazard. Preformed bromine chloride can also be used for this purpose.. Bromine Chloride Uses.
From www.bigstockphoto.com
Bromine Monochloride Image & Photo (Free Trial) Bigstock Bromine Chloride Uses Preformed bromine chloride can also be used for this purpose. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. Bromine(i) chloride is a chloride of bromine. Bromine monochloride, also. Bromine Chloride Uses.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Chloride Uses Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Risk assessment, environmental, and health hazard. Preformed bromine chloride can also be used for this purpose. Bromine(i) chloride is a. Bromine Chloride Uses.
From joiozxivu.blob.core.windows.net
Bromine Color Solution at Sharon Duran blog Bromine Chloride Uses Sushma, in hazardous gases, 2021. Bromine(i) chloride is a chloride of bromine. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Risk assessment, environmental, and health hazard. Toxic by. Bromine Chloride Uses.
From kunduz.com
[ANSWERED] Bromine monochloride is synthesized using the reaction Br g Bromine Chloride Uses Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Toxic by ingestion or. Bromine Chloride Uses.
From www.reddit.com
Chromyl chloride (left) vs. bromine (right). r/homechemistry Bromine Chloride Uses It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Risk assessment, environmental, and health hazard. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Bromine(i) chloride is a chloride of bromine.. Bromine Chloride Uses.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Chloride Uses Preformed bromine chloride can also be used for this purpose. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. Sushma, in hazardous gases, 2021. Bromine(i) chloride is a chloride of bromine. Risk assessment, environmental, and health hazard. Substitution reactions that use brcl are generally much faster than. Bromine Chloride Uses.
From www.google.com
EP1555879A1 Active bromine containing biocidal compositions and their Bromine Chloride Uses Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. Risk assessment, environmental, and health hazard. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Bromine(i) chloride is a chloride of bromine. Sushma, in hazardous gases, 2021. It is used as. Bromine Chloride Uses.
From www.differencebetween.com
Difference Between Bromine and Chlorine Compare the Difference Bromine Chloride Uses Substitution reactions that use brcl are generally much faster than those that use bromine alone. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Risk assessment, environmental, and health hazard. Sushma, in hazardous gases, 2021. Preformed bromine chloride can also be used for this purpose. Toxic by ingestion or inhalation, and an irritant. Bromine Chloride Uses.
From www.istockphoto.com
Bromine Monochloride Molecular Structure Isolated On White Stock Bromine Chloride Uses Risk assessment, environmental, and health hazard. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine(i) chloride is a chloride of bromine. Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous. Bromine Chloride Uses.
From www.chegg.com
Solved Under certain conditions, the substances bromine and Bromine Chloride Uses Risk assessment, environmental, and health hazard. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Preformed bromine chloride can also be used for this purpose. Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Bromine(i) chloride is a chloride of bromine. It is used as a biocide, specifically as. Bromine Chloride Uses.
From www.youtube.com
Bromine Water + Sodium Chloride YouTube Bromine Chloride Uses Substitution reactions that use brcl are generally much faster than those that use bromine alone. Sushma, in hazardous gases, 2021. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Risk assessment, environmental, and health hazard. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with.. Bromine Chloride Uses.
From www.slideserve.com
PPT Bromine PowerPoint Presentation, free download ID2319111 Bromine Chloride Uses Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Sushma, in hazardous gases, 2021. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Risk assessment, environmental, and health hazard. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction. Bromine Chloride Uses.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Chloride Uses Bromine(i) chloride is a chloride of bromine. Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Risk assessment, environmental, and health hazard. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling. Bromine Chloride Uses.
From techiescientist.com
BrCl Lewis Structure, Geometry, Hybridization, and Polarity Bromine Chloride Uses Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. Bromine(i) chloride is a chloride of bromine. Risk assessment, environmental, and health hazard. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Sushma, in hazardous gases, 2021. Toxic by ingestion or inhalation,. Bromine Chloride Uses.
From byjus.com
How do you test for an alkene? Bromine Chloride Uses Bromine(i) chloride is a chloride of bromine. Sushma, in hazardous gases, 2021. Preformed bromine chloride can also be used for this purpose. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals.. Bromine Chloride Uses.
From www.doubtnut.com
Bromine monochloride, (BrCl) into bromine and chlorine and Bromine Chloride Uses Bromine(i) chloride is a chloride of bromine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves. Bromine Chloride Uses.
From www.astonchem.com
Bromine chloride(BrCl3) (9CI) Bromine chloride(BrCl3) (9CI) 12360508 Bromine Chloride Uses Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. Risk assessment, environmental, and health hazard. Preformed bromine. Bromine Chloride Uses.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Bromine Chloride Uses Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine(i) chloride is a chloride of. Bromine Chloride Uses.
From www.youtube.com
How to find the Oxidation Number for Br in BrCl (Bromine monochloride Bromine Chloride Uses Sushma, in hazardous gases, 2021. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals.. Bromine Chloride Uses.
From www.slideserve.com
PPT Bromine PowerPoint Presentation, free download ID2813065 Bromine Chloride Uses Sushma, in hazardous gases, 2021. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Bromine(i) chloride is a chloride of bromine. Preformed bromine chloride can also be used for this purpose. Risk assessment, environmental, and health hazard. It. Bromine Chloride Uses.
From www.bigstockphoto.com
Bromine Monochloride Image & Photo (Free Trial) Bigstock Bromine Chloride Uses Substitution reactions that use brcl are generally much faster than those that use bromine alone. Bromine(i) chloride is a chloride of bromine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Preformed bromine chloride can also be used for this purpose. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride,. Bromine Chloride Uses.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica Bromine Chloride Uses It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Preformed bromine chloride can also be used for this purpose. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Toxic. Bromine Chloride Uses.
From www.thestudentroom.co.uk
Chemistry question OCR The Student Room Bromine Chloride Uses Sushma, in hazardous gases, 2021. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Risk assessment, environmental, and health hazard. Preformed bromine chloride can. Bromine Chloride Uses.
From www.alamy.com
Bromine monochloride hires stock photography and images Alamy Bromine Chloride Uses Sushma, in hazardous gases, 2021. Preformed bromine chloride can also be used for this purpose. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. Risk assessment, environmental, and health hazard. Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. It is used. Bromine Chloride Uses.
From hottubownerhq.com
What's the Difference Between Bromine and Chlorine? Hot Tub Owner HQ Bromine Chloride Uses Risk assessment, environmental, and health hazard. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Preformed bromine chloride can also be used for this purpose. Bromine(i) chloride is a chloride of bromine. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Bromine readily reacts. Bromine Chloride Uses.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Chloride Uses Bromine(i) chloride is a chloride of bromine. Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. Risk assessment, environmental, and health hazard. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Sushma, in hazardous gases, 2021. Bromine monochloride, also called bromine(i). Bromine Chloride Uses.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Bromine Chloride Uses It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Risk assessment, environmental, and health hazard. Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Bromine(i) chloride is a chloride of bromine.. Bromine Chloride Uses.
From woelen.homescience.net
Science made alive Chemistry/Experiments Bromine Chloride Uses Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Sushma, in hazardous gases, 2021. Preformed bromine chloride can also be used for this purpose. Bromine(i) chloride is a chloride of bromine. It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine monochloride, also called bromine(i) chloride,. Bromine Chloride Uses.
From www.dreamstime.com
BrCl Bromine Chloride CAS 13863417 Chemical Substance in White Bromine Chloride Uses Bromine readily reacts with saturated hydrocarbons and alkyl side chains of aromatic compounds through a chain reaction mechanism that involves free radicals. Sushma, in hazardous gases, 2021. Preformed bromine chloride can also be used for this purpose. Bromine(i) chloride is a chloride of bromine. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with.. Bromine Chloride Uses.
From www.alamy.com
Bubbling Chlorine Gas into Sodium Bromide to Yield Bromine Stock Photo Bromine Chloride Uses It is used as a biocide, specifically as an algaecide, fungicide, and disinfectant, in industrial recirculating cooling water. Bromine(i) chloride is a chloride of bromine. Sushma, in hazardous gases, 2021. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Risk. Bromine Chloride Uses.
From www.coursehero.com
Bromine monochloride is synthesized using the reaction Br2(g Bromine Chloride Uses Sushma, in hazardous gases, 2021. Bromine(i) chloride is a chloride of bromine. Substitution reactions that use brcl are generally much faster than those that use bromine alone. Bromine monochloride, also called bromine(i) chloride, bromochloride, and bromine chloride, is an interhalogen inorganic compound with. Toxic by ingestion or inhalation, and an irritant to skin, eyes and mucous membranes. Bromine readily reacts. Bromine Chloride Uses.