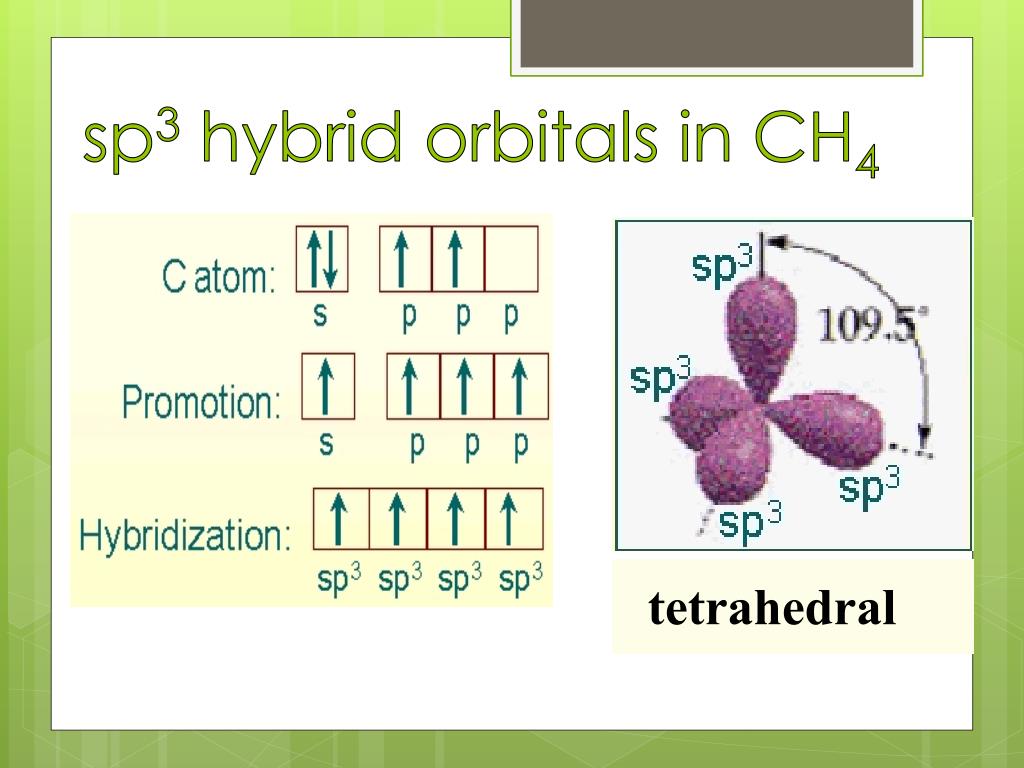
Hybridization Sp3 Examples . Find out the types of. Geometry, bond angles, shortcut + examples. The video below has a quick overview of. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). See examples of sp3 hybridization in molecules and ions, and how it. See the orbitals and electron. Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. Because carbon plays such a significant role in organic chemistry, we will be using it as an example. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. Sp 3 hybridization in methane; Sp hybrid orbitals are composed of one s and one p orbital. Learn about the mixing of one s orbital and three p orbitals to create four sp3 hybrid orbitals with tetrahedral geometry.
from www.slideserve.com
Sp 3 hybridization in methane; Find out the types of. Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. Geometry, bond angles, shortcut + examples. Because carbon plays such a significant role in organic chemistry, we will be using it as an example. See the orbitals and electron. See examples of sp3 hybridization in molecules and ions, and how it. Learn about the mixing of one s orbital and three p orbitals to create four sp3 hybrid orbitals with tetrahedral geometry. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). The video below has a quick overview of.
PPT Valence Bond Theory PowerPoint Presentation, free download ID
Hybridization Sp3 Examples Geometry, bond angles, shortcut + examples. The video below has a quick overview of. See examples of sp3 hybridization in molecules and ions, and how it. See the orbitals and electron. Because carbon plays such a significant role in organic chemistry, we will be using it as an example. Find out the types of. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). Geometry, bond angles, shortcut + examples. Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. Sp 3 hybridization in methane; Sp hybrid orbitals are composed of one s and one p orbital. Learn about the mixing of one s orbital and three p orbitals to create four sp3 hybrid orbitals with tetrahedral geometry.
From www.youtube.com
Hybridization sp3d sp3d2 sp3d3 Formation of PF5, SF6 and Hybridization Sp3 Examples Sp hybrid orbitals are composed of one s and one p orbital. See examples of sp3 hybridization in molecules and ions, and how it. The video below has a quick overview of. See the orbitals and electron. Sp 3 hybridization in methane; Geometry, bond angles, shortcut + examples. Learn about the mixing of one s orbital and three p orbitals. Hybridization Sp3 Examples.
From www.slideserve.com
PPT Valence Bond Theory PowerPoint Presentation, free download ID Hybridization Sp3 Examples Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. Find out the types of. Sp 3 hybridization in methane; Geometry, bond angles, shortcut + examples. The video below has a quick overview of. Learn about the concept of hybridization, the mixing of atomic orbitals to form. Hybridization Sp3 Examples.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Hybridization Sp3 Examples Find out the types of. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). See the orbitals and electron. Sp 3 hybridization in methane; Learn about the mixing of one s orbital. Hybridization Sp3 Examples.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Hybridization Sp3 Examples Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. Sp 3 hybridization in methane; Geometry, bond angles, shortcut + examples. See the orbitals and electron. Learn about the mixing of one s orbital and three p orbitals to create four sp3 hybrid orbitals with tetrahedral geometry.. Hybridization Sp3 Examples.
From chemistryteory.blogspot.com
chemistry Hybridization sp3 hybridization in Methane Hybridization Sp3 Examples Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. The video below has a quick overview of. See examples of sp3 hybridization in molecules and ions, and how it. Sp 3 hybridization in methane; Find out the types of. Learn about the mixing of one s. Hybridization Sp3 Examples.
From www.youtube.com
5.2BHybridization Examples sp sp2 sp3 YouTube Hybridization Sp3 Examples Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. Sp hybrid orbitals are composed of one s and one p orbital. Find out the types of. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds. Hybridization Sp3 Examples.
From mungfali.com
Sp3, Sp2, And Sp Hybridization In Organic Chemistry With Practice E19 Hybridization Sp3 Examples Geometry, bond angles, shortcut + examples. Because carbon plays such a significant role in organic chemistry, we will be using it as an example. Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. Find out the types of. Sp hybrid orbitals are composed of one s. Hybridization Sp3 Examples.
From www.chemistrylibrary.org
Sp3 hybridization Hybridization Sp3 Examples Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. See the orbitals and electron. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). See examples. Hybridization Sp3 Examples.
From www.youtube.com
5.2CHybridization Examples sp3d sp3d2 Lone Pairs YouTube Hybridization Sp3 Examples Sp 3 hybridization in methane; Find out the types of. Sp hybrid orbitals are composed of one s and one p orbital. Geometry, bond angles, shortcut + examples. Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. Because carbon plays such a significant role in organic. Hybridization Sp3 Examples.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Hybridization Sp3 Examples Because carbon plays such a significant role in organic chemistry, we will be using it as an example. Geometry, bond angles, shortcut + examples. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds. Hybridization Sp3 Examples.
From eduinput.com
sp3hybridization, definition, explanation, examples and significance Hybridization Sp3 Examples Find out the types of. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). See examples of sp3 hybridization in molecules and ions, and how it. The video below has a quick. Hybridization Sp3 Examples.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Hybridization Sp3 Examples Sp 3 hybridization in methane; See examples of sp3 hybridization in molecules and ions, and how it. Sp hybrid orbitals are composed of one s and one p orbital. Learn about the mixing of one s orbital and three p orbitals to create four sp3 hybrid orbitals with tetrahedral geometry. The video below has a quick overview of. Atoms with. Hybridization Sp3 Examples.
From general.chemistrysteps.com
sp, sp2, sp3, sp3d, and sp3d2 Hybridization Practice Problems Hybridization Sp3 Examples See examples of sp3 hybridization in molecules and ions, and how it. Because carbon plays such a significant role in organic chemistry, we will be using it as an example. Sp 3 hybridization in methane; Geometry, bond angles, shortcut + examples. Sp hybrid orbitals are composed of one s and one p orbital. Find out the types of. The video. Hybridization Sp3 Examples.
From chem.libretexts.org
Hybridization Chemistry LibreTexts Hybridization Sp3 Examples Geometry, bond angles, shortcut + examples. The video below has a quick overview of. Find out the types of. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). Learn about the mixing. Hybridization Sp3 Examples.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Hybridization Sp3 Examples Because carbon plays such a significant role in organic chemistry, we will be using it as an example. Learn about the mixing of one s orbital and three p orbitals to create four sp3 hybrid orbitals with tetrahedral geometry. Sp 3 hybridization in methane; Find out the types of. See examples of sp3 hybridization in molecules and ions, and how. Hybridization Sp3 Examples.
From www.geeksforgeeks.org
Hybridization Definition, Types, Rules, Examples Hybridization Sp3 Examples Find out the types of. Sp hybrid orbitals are composed of one s and one p orbital. Learn about the mixing of one s orbital and three p orbitals to create four sp3 hybrid orbitals with tetrahedral geometry. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. Geometry, bond angles, shortcut + examples.. Hybridization Sp3 Examples.
From leah4sci.com
Sp3, Sp2 and Sp Hybridization, Geometry and Bond Angles Hybridization Sp3 Examples The video below has a quick overview of. Learn about the mixing of one s orbital and three p orbitals to create four sp3 hybrid orbitals with tetrahedral geometry. Because carbon plays such a significant role in organic chemistry, we will be using it as an example. Geometry, bond angles, shortcut + examples. Learn about the concept of hybridization, the. Hybridization Sp3 Examples.
From www.youtube.com
How to determine Hybridization s, sp, sp2, and sp3 Organic Hybridization Sp3 Examples Find out the types of. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). The video below has a quick overview of. Geometry, bond angles, shortcut + examples. Because carbon plays such. Hybridization Sp3 Examples.
From www.adda247.com
sp, sp2, sp3 Hybridization Examples, sp3d2 Shape & Structure Hybridization Sp3 Examples See the orbitals and electron. Sp hybrid orbitals are composed of one s and one p orbital. Because carbon plays such a significant role in organic chemistry, we will be using it as an example. See examples of sp3 hybridization in molecules and ions, and how it. Learn about the concept of hybridization, the mixing of atomic orbitals to form. Hybridization Sp3 Examples.
From bunchostel.com
Hybridization of Atomic Orbitals Sigma & Pi Bonds Sp Sp2 Sp3 sp3 Hybridization Sp3 Examples Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). See examples of sp3 hybridization in molecules and ions, and how it. Sp hybrid orbitals are composed of one s and one p. Hybridization Sp3 Examples.
From ar.inspiredpencil.com
Sp3 Hybridization Hybridization Sp3 Examples Find out the types of. Sp hybrid orbitals are composed of one s and one p orbital. See examples of sp3 hybridization in molecules and ions, and how it. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. Geometry, bond angles, shortcut + examples. Learn about the mixing of one s orbital and. Hybridization Sp3 Examples.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Hybridization Sp3 Examples See examples of sp3 hybridization in molecules and ions, and how it. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). Learn about the mixing of one s orbital and three p. Hybridization Sp3 Examples.
From glossary.periodni.com
Sp3 hybrid orbital Chemistry Dictionary & Glossary Hybridization Sp3 Examples See examples of sp3 hybridization in molecules and ions, and how it. Sp hybrid orbitals are composed of one s and one p orbital. Because carbon plays such a significant role in organic chemistry, we will be using it as an example. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. Geometry, bond. Hybridization Sp3 Examples.
From www.youtube.com
How to Determine the Hybridization of an Atom (sp, sp2, sp3, sp3d Hybridization Sp3 Examples See examples of sp3 hybridization in molecules and ions, and how it. Sp 3 hybridization in methane; Geometry, bond angles, shortcut + examples. Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. Because carbon plays such a significant role in organic chemistry, we will be using. Hybridization Sp3 Examples.
From madoverchemistry.com
62.CHEMICAL BONDING (9) Covalent Bonding(8) sp3 Hybridization Hybridization Sp3 Examples See the orbitals and electron. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). Find out the types of. Learn about the concept of hybridization, the mixing of atomic orbitals to form. Hybridization Sp3 Examples.
From www.youtube.com
SP3 HYBRIDIZATION_ PART 01 YouTube Hybridization Sp3 Examples The video below has a quick overview of. Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. Sp hybrid orbitals are composed of one s and one p orbital. Because carbon plays such a significant role in organic chemistry, we will be using it as an. Hybridization Sp3 Examples.
From chemistrytalk.org
Hybridization and Hybrid Orbitals ChemTalk Hybridization Sp3 Examples Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. Geometry, bond angles, shortcut + examples. The video below has a quick overview of. See the orbitals and electron. Sp 3 hybridization in methane; Because carbon plays such a significant role in organic chemistry, we will be. Hybridization Sp3 Examples.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Hybridization Sp3 Examples Because carbon plays such a significant role in organic chemistry, we will be using it as an example. The video below has a quick overview of. Learn about the mixing of one s orbital and three p orbitals to create four sp3 hybrid orbitals with tetrahedral geometry. Find out the types of. Atoms with sp hybridization have two leftover unhybridized. Hybridization Sp3 Examples.
From chemistnotes.com
Hybridization Definition, types and examples Chemistry Notes Hybridization Sp3 Examples Sp hybrid orbitals are composed of one s and one p orbital. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). See examples of sp3 hybridization in molecules and ions, and how. Hybridization Sp3 Examples.
From collegedunia.com
Hybridization sp, sp2, sp3 & sp3d Atomic Orbitals, Properties & Examples Hybridization Sp3 Examples Because carbon plays such a significant role in organic chemistry, we will be using it as an example. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). Learn about the concept of. Hybridization Sp3 Examples.
From www.youtube.com
sp3, sp2, and sp Hybridization YouTube Hybridization Sp3 Examples See the orbitals and electron. Find out the types of. Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. The video below has a quick overview of. Geometry, bond angles, shortcut + examples. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in. Hybridization Sp3 Examples.
From www.pinterest.com
Image result for hybridization sp sp2 sp3 and shape Molecular shapes Hybridization Sp3 Examples Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. See the orbitals and electron. The video below has a quick overview of. Find out the types of. Sp 3 hybridization in methane; Learn about the mixing of one s orbital and three p orbitals to create four sp3 hybrid orbitals with tetrahedral geometry.. Hybridization Sp3 Examples.
From www.globalsino.com
sp3 Hybridization Hybridization Sp3 Examples Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, most often as triple bonds (although “2+2” bonding is also seen, like in co 2 or organic molecules called ketenes, for example). Learn about the mixing of one s orbital and three p orbitals to create four sp3 hybrid orbitals with tetrahedral geometry. Geometry, bond. Hybridization Sp3 Examples.
From www.pinterest.com.mx
Hybridizationsp3,sp2,sp hybridization Hybridization of carbon Hybridization Sp3 Examples Geometry, bond angles, shortcut + examples. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen and other atoms in methane, ethane, ammonia, and water. See examples of sp3 hybridization in molecules and ions, and how it. See the orbitals. Hybridization Sp3 Examples.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Hybridization Sp3 Examples See the orbitals and electron. Sp hybrid orbitals are composed of one s and one p orbital. Find out the types of. Because carbon plays such a significant role in organic chemistry, we will be using it as an example. Geometry, bond angles, shortcut + examples. Learn how carbon and nitrogen atoms form sp³ hybrid orbitals and bond with hydrogen. Hybridization Sp3 Examples.