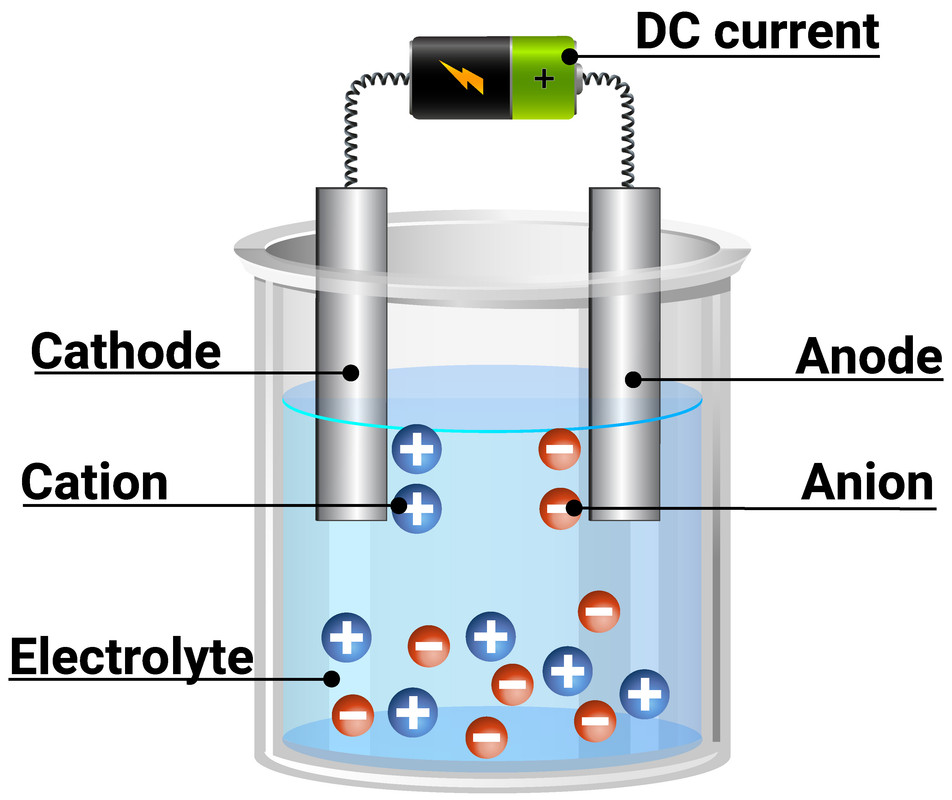
Electrolysis Fluid . The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. An external voltage is applied to drive a nonspontaneous. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The electrolytic cell consists of a pair of platinum electrodes immersed in water.
from www.revisechemistry.uk
Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The electrolysis of water produces hydrogen and oxygen gases. An external voltage is applied to drive a nonspontaneous. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The electrolytic cell consists of a pair of platinum electrodes immersed in water.
Electrolysis OCR Gateway C3 revisechemistry.uk
Electrolysis Fluid Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. The electrolysis of water produces hydrogen and oxygen gases. An external voltage is applied to drive a nonspontaneous. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The electrolytic cell consists of a pair of platinum electrodes immersed in water.
From www.researchgate.net
Configurations for water electrolysis (a) proton exchange membrane Electrolysis Fluid Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs:. Electrolysis Fluid.
From www.chemistrylearner.com
Electrolysis of Water Definition and Equation Electrolysis Fluid The electrolytic cell consists of a pair of platinum electrodes immersed in water. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. An external voltage is applied to drive a nonspontaneous. The. Electrolysis Fluid.
From mavink.com
Labelled Diagram Of Electrolysis Electrolysis Fluid Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and. Electrolysis Fluid.
From www.youtube.com
Electrolysis Tank Basics YouTube Electrolysis Fluid Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. In an electrolytic cell, however, the opposite process, called electrolysis,. Electrolysis Fluid.
From www.aakash.ac.in
Electrolysis Definition, Mechanism, Meaning & Application Chemistry Electrolysis Fluid The electrolytic cell consists of a pair of platinum electrodes immersed in water. The electrolysis of water produces hydrogen and oxygen gases. An external voltage is applied to drive a nonspontaneous. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Water electrolysis. Electrolysis Fluid.
From byjus.com
Water Electrolysis Principle of Water Electrolysis, Important Factors Electrolysis Fluid Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. The electrolytic cell consists of a pair of platinum electrodes immersed in water. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: An external voltage is applied to drive a nonspontaneous. Electrolysis, process by which electric current is. Electrolysis Fluid.
From www.dreamstime.com
Electrolysis of Electrolyte Solution in Electrochemistry Vector Electrolysis Fluid Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. The electrolysis of water produces hydrogen and oxygen gases. In an. Electrolysis Fluid.
From www.researchgate.net
Schematic diagram of electrolysis process. Download Scientific Diagram Electrolysis Fluid The electrolysis of water produces hydrogen and oxygen gases. An external voltage is applied to drive a nonspontaneous. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Electrolysis, process by which electric current is passed. Electrolysis Fluid.
From www.alamy.com
Electrolysis process vector illustration. Simple electrolysis process Electrolysis Fluid Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. The electrolysis of water produces hydrogen and oxygen gases. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. An external voltage is applied to drive a nonspontaneous. Water electrolysis is the most significant. Electrolysis Fluid.
From www2.mdpi.com
Processes Free FullText CFD Modeling and Experimental Validation Electrolysis Fluid The electrolysis of water produces hydrogen and oxygen gases. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. An external voltage is applied to drive a nonspontaneous. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Water electrolysis is an electrochemical process in which electricity is used to split. Electrolysis Fluid.
From enagic-asia.com
The electrolysis process Enagic Kangen Water Electrolysis Fluid An external voltage is applied to drive a nonspontaneous. The electrolytic cell consists of a pair of platinum electrodes immersed in water. The electrolysis of water produces hydrogen and oxygen gases. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen. Electrolysis Fluid.
From www.parker.com
Electrolysis Flow Chart Parker Electrolysis Fluid Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Electrolysis, process by which electric current. Electrolysis Fluid.
From study.com
Faraday's Laws of Electrolysis Equation & Constant Units Lesson Electrolysis Fluid The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Water electrolysis is an electrochemical process in which electricity is used to split water into two gases,. Electrolysis Fluid.
From www.freepik.com
Premium Vector Electrolysis process vector illustration diagram Electrolysis Fluid Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. An external voltage is applied to drive a nonspontaneous. The electrolytic cell consists. Electrolysis Fluid.
From energy.gov
Hydrogen Production Electrolysis Department of Energy Electrolysis Fluid Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: An external voltage is applied to drive a nonspontaneous. The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is an electrochemical process in which electricity is used to split water into. Electrolysis Fluid.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Electrolysis Fluid The electrolytic cell consists of a pair of platinum electrodes immersed in water. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. An external voltage is applied to. Electrolysis Fluid.
From www.researchgate.net
Experimental set up of HEFR (hybrid of mixed electrolysis and fluids Electrolysis Fluid Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. Water electrolysis is the most significant primary electrochemical method for hydrogen. Electrolysis Fluid.
From morrisclassicalacademy.blogspot.com
Morris Classical Academy Electrolysis of water Electrolysis Fluid The electrolytic cell consists of a pair of platinum electrodes immersed in water. Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. The electrolysis of water produces hydrogen and oxygen gases. An external voltage is applied to drive a nonspontaneous. Water electrolysis is the most significant primary electrochemical method. Electrolysis Fluid.
From www.linkedin.com
Water Electrolysis Different Methods Electrolysis Fluid Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. An external voltage is applied to drive a nonspontaneous. The electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes immersed in water. Electrolysis, process by which electric current is passed. Electrolysis Fluid.
From www.energy.dtu.dk
How does electrolysis work Electrolysis Fluid The electrolytic cell consists of a pair of platinum electrodes immersed in water. An external voltage is applied to drive a nonspontaneous. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly. Electrolysis Fluid.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolysis Fluid Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The electrolysis of water produces hydrogen and oxygen gases. An external voltage. Electrolysis Fluid.
From mungfali.com
Electrolysis Process Diagram Electrolysis Fluid Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. In an electrolytic cell, however, the opposite process, called electrolysis,. Electrolysis Fluid.
From www.vecteezy.com
Electrolysis of Sodium Chloride solution 18891988 Vector Art at Vecteezy Electrolysis Fluid An external voltage is applied to drive a nonspontaneous. Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. The electrolytic cell consists of a pair of platinum electrodes immersed in water. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The electrolysis of water produces hydrogen and. Electrolysis Fluid.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at Electrolysis Fluid The electrolysis of water produces hydrogen and oxygen gases. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The electrolytic cell consists of a pair of platinum electrodes immersed in water. An external voltage is applied to drive a nonspontaneous. Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen. Electrolysis Fluid.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Electrolysis Fluid Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. The electrolytic cell consists of a pair of platinum electrodes immersed in water. An external voltage is applied to drive a nonspontaneous. In. Electrolysis Fluid.
From mungfali.com
Diagram Of Electrolysis Electrolysis Fluid The electrolysis of water produces hydrogen and oxygen gases. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The electrolytic cell consists of a pair of platinum electrodes immersed in water. An external voltage is applied to drive a nonspontaneous. Water electrolysis. Electrolysis Fluid.
From www.youtube.com
Chemistry and Double Investigation Copper Electrolysis 2016 YouTube Electrolysis Fluid The electrolysis of water produces hydrogen and oxygen gases. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The electrolytic cell. Electrolysis Fluid.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolysis Fluid Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes immersed in water. In. Electrolysis Fluid.
From www.researchgate.net
Flexible Electrolyzer Design of Decoupled Water Electrolysis (A Electrolysis Fluid The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. In an electrolytic cell, however, the opposite process, called electrolysis,. Electrolysis Fluid.
From unitedhydrogenlimited.com
What is electrolysis? United H2 Limited Electrolysis Fluid Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. The electrolytic cell consists of a pair of platinum electrodes immersed in water. An external voltage is applied to drive a nonspontaneous. Water electrolysis is an electrochemical process in which electricity is used to split water into two gases,. Electrolysis Fluid.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Electrolysis Fluid Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The electrolysis of water produces hydrogen and oxygen gases. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Water electrolysis is the. Electrolysis Fluid.
From study.com
Electrolysis of Aqueous Solutions Lesson Electrolysis Fluid The electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes immersed in water. An external voltage is applied to drive a nonspontaneous. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. Water electrolysis is an electrochemical process in which. Electrolysis Fluid.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Electrolysis Fluid Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly with renewable energy. The electrolysis of water produces hydrogen and oxygen gases. An external voltage is applied to drive a nonspontaneous. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis, process by which electric current is passed through a. Electrolysis Fluid.
From mavink.com
Labelled Diagram Of Electrolysis Electrolysis Fluid An external voltage is applied to drive a nonspontaneous. The electrolytic cell consists of a pair of platinum electrodes immersed in water. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The electrolysis of water produces hydrogen and oxygen gases. Water electrolysis is the most significant primary electrochemical method for hydrogen production, and its importance will increase rapidly. Electrolysis Fluid.
From books.studentsource.org
Electrolysis Electrolysis Fluid The electrolytic cell consists of a pair of platinum electrodes immersed in water. The electrolysis of water produces hydrogen and oxygen gases. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Water electrolysis is an electrochemical process in which electricity is used to split water into two gases, hydrogen and oxygen. An external voltage is applied to drive. Electrolysis Fluid.