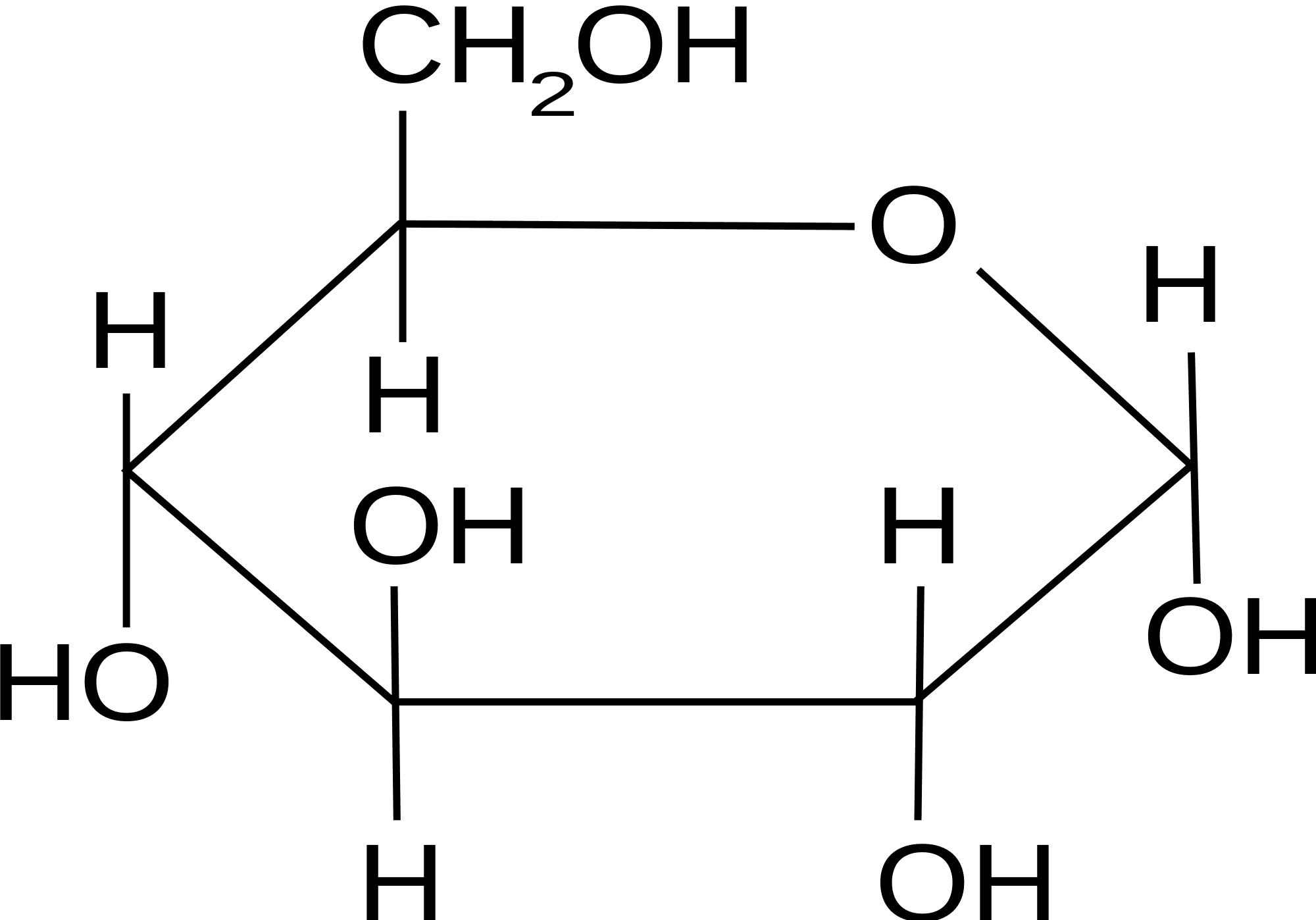
Cyclic Chain Structure Of Glucose . These are known as anomers. When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). In an aqueous solution, an equilibrium mixture forms between the two. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. Glycoside formation confirms cyclic structure: The α form melts at 146°c and has a specific. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure.
from www.lookfordiagnosis.com
It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). In an aqueous solution, an equilibrium mixture forms between the two. The α form melts at 146°c and has a specific. These are known as anomers. Glycoside formation confirms cyclic structure: It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals.
Glucose; dextrose
Cyclic Chain Structure Of Glucose In an aqueous solution, an equilibrium mixture forms between the two. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. Glycoside formation confirms cyclic structure: When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. These are known as anomers. The α form melts at 146°c and has a specific. In an aqueous solution, an equilibrium mixture forms between the two.
From www.dreamstime.com
Glucose. Grape Sugar or Dextrose. Monosaccharide. Cyclic and Open Chain Cyclic Chain Structure Of Glucose Glycoside formation confirms cyclic structure: It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. The α form melts at 146°c and has a specific. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). These are known. Cyclic Chain Structure Of Glucose.
From ar.inspiredpencil.com
Structure Of Glucose Cyclic Chain Structure Of Glucose In an aqueous solution, an equilibrium mixture forms between the two. The α form melts at 146°c and has a specific. These are known as anomers. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. When we use methanol in the presence of dry. Cyclic Chain Structure Of Glucose.
From www.researchgate.net
Equilibrium reaction schemes of (a) Dglucose and (b) Dribose Cyclic Chain Structure Of Glucose The α form melts at 146°c and has a specific. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. These are known as anomers. Glycoside formation confirms cyclic structure: In an aqueous solution, an equilibrium mixture forms between the two. It is possible to. Cyclic Chain Structure Of Glucose.
From xkldase.edu.vn
Share 122+ ring form of glucose super hot xkldase.edu.vn Cyclic Chain Structure Of Glucose These are known as anomers. In an aqueous solution, an equilibrium mixture forms between the two. The α form melts at 146°c and has a specific. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. One of the most important carbohydrates in the body. Cyclic Chain Structure Of Glucose.
From jackwestin.com
Cyclic Structure And Conformations Of Hexoses Carbohydrates MCAT Cyclic Chain Structure Of Glucose In an aqueous solution, an equilibrium mixture forms between the two. Glycoside formation confirms cyclic structure: These are known as anomers. The α form melts at 146°c and has a specific. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. When we use methanol. Cyclic Chain Structure Of Glucose.
From ar.inspiredpencil.com
Glucose Ring Structure Formation Cyclic Chain Structure Of Glucose It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. The α form melts at 146°c and has a specific. These are known as anomers. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). In an aqueous. Cyclic Chain Structure Of Glucose.
From www.wou.edu
CH103 Chapter 8 The Major Macromolecules Chemistry Cyclic Chain Structure Of Glucose It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. These are known as anomers. When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. Glycoside formation confirms cyclic structure: The α form melts at. Cyclic Chain Structure Of Glucose.
From testbook.com
Glucose Structure Understanding Different Forms, OpenChain Formula Cyclic Chain Structure Of Glucose One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). In an aqueous solution, an equilibrium mixture forms between the two. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. These are known as anomers. Glycoside formation. Cyclic Chain Structure Of Glucose.
From surfguppy.com
4 simple steps to drawing chain structure of glucose molecule Cyclic Chain Structure Of Glucose When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. Glycoside formation confirms cyclic structure: It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. These are known as anomers. The α form melts at. Cyclic Chain Structure Of Glucose.
From www.pinterest.co.uk
glucose cyclic structure Chemistry lecture, Chemistry textbook Cyclic Chain Structure Of Glucose These are known as anomers. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. The α form melts at 146°c and has a specific. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or. Cyclic Chain Structure Of Glucose.
From www.alamy.com
Glucose (dextrose, Dglucose) molecule. Сyclic and acyclic forms. Sheet Cyclic Chain Structure Of Glucose When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. Glycoside formation confirms cyclic structure: The α form melts at 146°c and has a specific. These are known as anomers. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or. Cyclic Chain Structure Of Glucose.
From byjus.com
Classification of Carbohydrates with Types, Formula and Structure Cyclic Chain Structure Of Glucose The α form melts at 146°c and has a specific. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). In an aqueous solution, an equilibrium mixture forms between the two. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have. Cyclic Chain Structure Of Glucose.
From www.youtube.com
Trick to draw Cyclic Structure of Glucose class 12 Biomolecules JEE Cyclic Chain Structure Of Glucose The α form melts at 146°c and has a specific. In an aqueous solution, an equilibrium mixture forms between the two. When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. These are known as anomers. It is possible to obtain a sample of crystalline glucose in which all the. Cyclic Chain Structure Of Glucose.
From surfguppy.com
4 simple steps to drawing chain structure of glucose molecule Cyclic Chain Structure Of Glucose It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). These are known as anomers. The α form melts at 146°c and has a specific. When we use. Cyclic Chain Structure Of Glucose.
From surfguppy.com
3 Simple Steps Draw the ring structure of glucose molecule Cyclic Chain Structure Of Glucose The α form melts at 146°c and has a specific. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. In an aqueous solution, an equilibrium mixture forms between the two. Glycoside formation confirms cyclic structure: When we use methanol in the presence of dry. Cyclic Chain Structure Of Glucose.
From byjus.com
Glucose Structure Diagrams, Examples, Physical Properties Cyclic Chain Structure Of Glucose It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. These are known as anomers. Glycoside formation confirms cyclic structure: One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). When we use methanol in the presence of. Cyclic Chain Structure Of Glucose.
From byjus.com
Classification of Carbohydrates Carbohydrate Definition, Types of Cyclic Chain Structure Of Glucose These are known as anomers. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. Glycoside formation confirms cyclic structure: It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure.. Cyclic Chain Structure Of Glucose.
From www.alamy.com
Glucose, monosaccharide, chemical structure. Simple sugar. Natta Cyclic Chain Structure Of Glucose It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. The α form melts at 146°c and has a specific. Glycoside formation confirms cyclic structure: These are known as anomers. In an aqueous solution, an equilibrium mixture forms between the two. One of the most. Cyclic Chain Structure Of Glucose.
From healthjade.com
What is Carbohydrates? Foods, Healthy Carbs for Weight Loss Cyclic Chain Structure Of Glucose When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. The α form melts at 146°c and has a specific. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). In an aqueous solution, an equilibrium mixture forms between the two. Glycoside formation. Cyclic Chain Structure Of Glucose.
From www.vedantu.com
The open chain structure of glucose was proposed by (A) Lobry de bruyn Cyclic Chain Structure Of Glucose The α form melts at 146°c and has a specific. Glycoside formation confirms cyclic structure: It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or. Cyclic Chain Structure Of Glucose.
From www.doubtnut.com
Write the reactions of Dglucose which can't be explained by its open Cyclic Chain Structure Of Glucose One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. In an aqueous solution, an equilibrium mixture forms between the two. When we use methanol in the presence. Cyclic Chain Structure Of Glucose.
From chem.libretexts.org
4.3 32 Conformations of cyclic organic molecules Chemistry LibreTexts Cyclic Chain Structure Of Glucose These are known as anomers. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. Glycoside formation confirms cyclic structure: In an aqueous solution, an equilibrium mixture forms between the two. It is. Cyclic Chain Structure Of Glucose.
From www.lookfordiagnosis.com
Glucose; dextrose Cyclic Chain Structure Of Glucose The α form melts at 146°c and has a specific. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. These are known as anomers. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or. Cyclic Chain Structure Of Glucose.
From tropicalcyclocross.com
Glucose Chain Structure Cyclic Chain Structure Of Glucose When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). These are known as anomers. Glycoside formation confirms cyclic structure: It is possible to obtain a sample of crystalline glucose in which all. Cyclic Chain Structure Of Glucose.
From www.studypool.com
SOLUTION D(+)GLUCOSE Chemical Properties and Structure, Open Chain Cyclic Chain Structure Of Glucose It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. Glycoside formation confirms cyclic structure: When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. It is possible to obtain a sample of crystalline glucose. Cyclic Chain Structure Of Glucose.
From alevelbiology.co.uk
Glucose Structure, Properties, Synthesis, Facts & Summary Cyclic Chain Structure Of Glucose When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. These are known as anomers. Glycoside formation confirms cyclic structure: In an aqueous solution, an equilibrium mixture forms between the two. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). The α. Cyclic Chain Structure Of Glucose.
From www.mz-store.com
Information about carbohydrates Cyclic Chain Structure Of Glucose It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). These are known as anomers. Glycoside formation confirms cyclic structure: The α form melts at 146°c and has. Cyclic Chain Structure Of Glucose.
From ar.inspiredpencil.com
Structure Of Glucose Cyclic Chain Structure Of Glucose It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. In an aqueous solution, an equilibrium mixture forms between the two. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β. Cyclic Chain Structure Of Glucose.
From www.chegg.com
Solved Two structures of glucose are shown here. Cyclic Chain Structure Of Glucose One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). The α form melts at 146°c and has a specific. When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. It is possible to obtain a sample of crystalline glucose in which all. Cyclic Chain Structure Of Glucose.
From byjus.com
Explain the ring structure which is involving in the cyclic structure Cyclic Chain Structure Of Glucose It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). The α form melts at 146°c and has a specific. In an aqueous solution, an equilibrium mixture forms. Cyclic Chain Structure Of Glucose.
From gbu-presnenskij.ru
Structure Of Glucose, Straight Chain Structure Of Glucose,, 57 OFF Cyclic Chain Structure Of Glucose One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). Glycoside formation confirms cyclic structure: It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. In an aqueous solution, an equilibrium mixture forms between the two. The α. Cyclic Chain Structure Of Glucose.
From lookfordiagnosis.com
D glucose Cyclic Chain Structure Of Glucose One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). These are known as anomers. When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. In an aqueous solution, an equilibrium mixture forms between the two. It is possible to obtain a sample. Cyclic Chain Structure Of Glucose.
From www.animalia-life.club
Structure Of Glucose Fructose And Sucrose Cyclic Chain Structure Of Glucose It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure. Glycoside formation confirms cyclic structure: One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). It is possible to obtain a sample of crystalline glucose in which all. Cyclic Chain Structure Of Glucose.
From tropicalcyclocross.com
Glucose Chain Structure Cyclic Chain Structure Of Glucose The α form melts at 146°c and has a specific. Glycoside formation confirms cyclic structure: When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. It is possible to obtain a sample of crystalline glucose in which all the molecules have the α structure or all have the β structure.. Cyclic Chain Structure Of Glucose.
From chem.libretexts.org
A1. Monosaccharides Structures Chemistry LibreTexts Cyclic Chain Structure Of Glucose One of the most important carbohydrates in the body is glucose (c 6 h 12 o 6). When we use methanol in the presence of dry hcl to treat glucose, it gives two isomeric glycosides or acetals. In an aqueous solution, an equilibrium mixture forms between the two. These are known as anomers. It is possible to obtain a sample. Cyclic Chain Structure Of Glucose.