Negative Standard Enthalpy Of Formation Will Be Maximum For . The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. This is the commonest use of simple hess's law cycles that you are likely to come across. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. In this case, we are going to calculate the enthalpy change for the reaction between ethene and. 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is.
from www.youtube.com
The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. In this case, we are going to calculate the enthalpy change for the reaction between ethene and. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can exist as ozone (o 3. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. This is the commonest use of simple hess's law cycles that you are likely to come across. 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is.
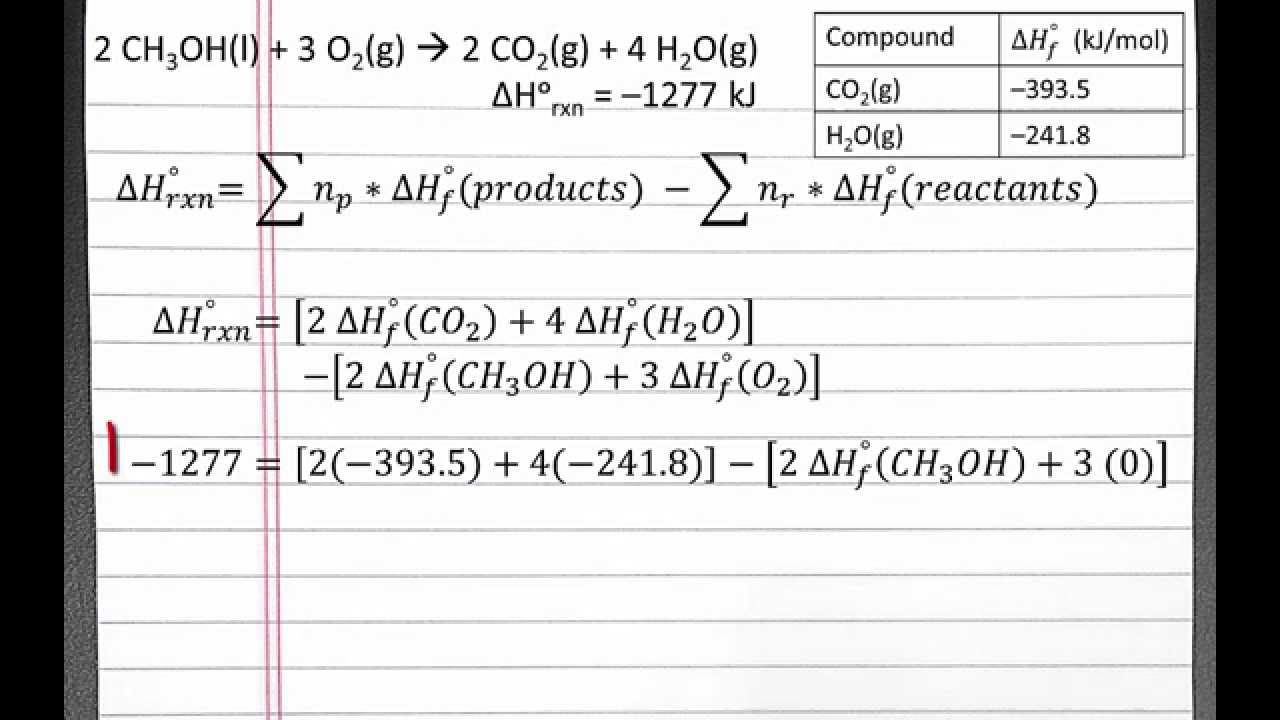
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies
Negative Standard Enthalpy Of Formation Will Be Maximum For The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. For example, although oxygen can exist as ozone (o 3. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. In this case, we are going to calculate the enthalpy change for the reaction between ethene and. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This is the commonest use of simple hess's law cycles that you are likely to come across. The standard enthalpy of formation of any element in its standard state is zero by definition.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Negative Standard Enthalpy Of Formation Will Be Maximum For By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. This is the commonest use of simple hess's law cycles that you are likely to come across. In this case, we are going to calculate the enthalpy change for the reaction between ethene. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Negative Standard Enthalpy Of Formation Will Be Maximum For By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From studylib.net
I. Standard Enthalpies of Formation Negative Standard Enthalpy Of Formation Will Be Maximum For The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is. The standard enthalpy of formation is given the symbol δfho δ f. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.youtube.com
Standard Enthalpies of Formation Problem (Chemistry) YouTube Negative Standard Enthalpy Of Formation Will Be Maximum For A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Negative Standard Enthalpy Of Formation Will Be Maximum For 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation is given the symbol δfho δ f. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Negative Standard Enthalpy Of Formation Will Be Maximum For The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This is the commonest use of simple hess's law cycles that you are likely to come across. In this case, we are going to calculate the enthalpy change for the reaction between ethene and. For example,. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Negative Standard Enthalpy Of Formation Will Be Maximum For For example, although oxygen can exist as ozone (o 3. 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is. In this case, we are going to calculate the enthalpy change for the reaction between ethene and. A standard enthalpy of formation $δh°_f$ is an enthalpy. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Negative Standard Enthalpy Of Formation Will Be Maximum For The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Negative Standard Enthalpy Of Formation Will Be Maximum For 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is given the symbol δfho. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.chegg.com
Solved 8) a) Define "standard enthalpy change of formation." Negative Standard Enthalpy Of Formation Will Be Maximum For This is the commonest use of simple hess's law cycles that you are likely to come across. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can exist as ozone (o 3. The standard enthalpy of formation of any. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Negative Standard Enthalpy Of Formation Will Be Maximum For By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.slideshare.net
Standard enthalpy of formation Negative Standard Enthalpy Of Formation Will Be Maximum For This is the commonest use of simple hess's law cycles that you are likely to come across. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.nagwa.com
Question Video Using Standard Enthalpies of Formation to Find Δ퐻⦵ in Negative Standard Enthalpy Of Formation Will Be Maximum For A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. This is the commonest use of simple. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.youtube.com
Standard Enthalpy of Formation Thermodynamics Heat, Enthalpy Negative Standard Enthalpy Of Formation Will Be Maximum For The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. In this case, we are going to calculate the enthalpy change for the reaction between ethene and. 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5072129 Negative Standard Enthalpy Of Formation Will Be Maximum For By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is. In this case, we are going to calculate the. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Negative Standard Enthalpy Of Formation Will Be Maximum For The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3. This is the commonest use of simple hess's law cycles. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Negative Standard Enthalpy Of Formation Will Be Maximum For This is the commonest use of simple hess's law cycles that you are likely to come across. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. In this case, we are going to calculate the enthalpy change for the reaction between ethene and. A standard enthalpy of. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME Negative Standard Enthalpy Of Formation Will Be Maximum For 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. By definition, the standard enthalpy of formation of an element in its. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Negative Standard Enthalpy Of Formation Will Be Maximum For The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. This is the commonest use of simple hess's law cycles that you are likely to come across. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows if the standard. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.chemistryspace.com
Standard Enthalpy of Formation Negative Standard Enthalpy Of Formation Will Be Maximum For This is the commonest use of simple hess's law cycles that you are likely to come across. 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.slideserve.com
PPT Energy & Chemical Change PowerPoint Presentation, free download Negative Standard Enthalpy Of Formation Will Be Maximum For For example, although oxygen can exist as ozone (o 3. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. 193 rows if the. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.toppr.com
Calculate the standard enthalpy of formation of CH3 OH from the Negative Standard Enthalpy Of Formation Will Be Maximum For For example, although oxygen can exist as ozone (o 3. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. In this case, we are going to calculate the enthalpy change for the reaction between ethene and. The standard enthalpy of formation of any. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Negative Standard Enthalpy Of Formation Will Be Maximum For The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. In this case, we are going to calculate the enthalpy change for the reaction between ethene and. For example, although oxygen can exist as ozone (o 3. The standard enthalpy of formation of any element in its standard. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From mavink.com
Enthalpy Of Formation Equation Negative Standard Enthalpy Of Formation Will Be Maximum For This is the commonest use of simple hess's law cycles that you are likely to come across. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Negative Standard Enthalpy Of Formation Will Be Maximum For By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. This is the commonest use of simple hess's law cycles that you are likely to come across. The standard enthalpy of formation is a measure of the energy released or consumed when one. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From scienceinfo.com
Enthalpy Introduction, Calculation, Enthalpy change, Importance Negative Standard Enthalpy Of Formation Will Be Maximum For By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation is given the symbol. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Negative Standard Enthalpy Of Formation Will Be Maximum For The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. In this case, we are going to calculate the enthalpy change for the reaction between ethene and. The standard enthalpy of formation of any element in its standard state is zero by definition. This is the commonest use. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Negative Standard Enthalpy Of Formation Will Be Maximum For The standard enthalpy of formation of any element in its standard state is zero by definition. In this case, we are going to calculate the enthalpy change for the reaction between ethene and. For example, although oxygen can exist as ozone (o 3. By definition, the standard enthalpy of formation of an element in its most stable form is equal. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Negative Standard Enthalpy Of Formation Will Be Maximum For 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. By definition, the standard enthalpy of formation of an element. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From studylib.net
Standard Enthalpy of Formation Negative Standard Enthalpy Of Formation Will Be Maximum For This is the commonest use of simple hess's law cycles that you are likely to come across. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.chegg.com
Solved Calculate the standard enthalpy of formation of Negative Standard Enthalpy Of Formation Will Be Maximum For For example, although oxygen can exist as ozone (o 3. This is the commonest use of simple hess's law cycles that you are likely to come across. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Negative Standard Enthalpy Of Formation Will Be Maximum For The standard enthalpy of formation of any element in its standard state is zero by definition. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From studylib.net
Standard Enthalpy of Formation and Reaction Negative Standard Enthalpy Of Formation Will Be Maximum For 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is. This is the commonest use of simple hess's law cycles that you are likely to come across. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Negative Standard Enthalpy Of Formation Will Be Maximum For 193 rows if the standard enthalpy of the products is less than the standard enthalpy of the reactants, the standard enthalpy of reaction is. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can exist as ozone (o 3.. Negative Standard Enthalpy Of Formation Will Be Maximum For.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Negative Standard Enthalpy Of Formation Will Be Maximum For This is the commonest use of simple hess's law cycles that you are likely to come across. For example, although oxygen can exist as ozone (o 3. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript. Negative Standard Enthalpy Of Formation Will Be Maximum For.