Spectral Lines Definition Chemistry . A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. The reason the chemical elements can do this is. The lines that a particular element produces are called the emission spectrum of that element. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements.
from studylib.net
A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. The lines that a particular element produces are called the emission spectrum of that element. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. The reason the chemical elements can do this is. Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and.
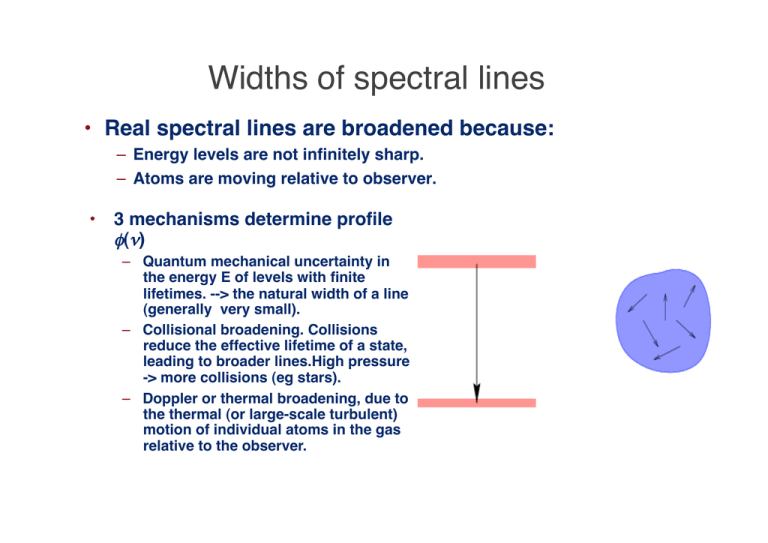
Widths of spectral lines
Spectral Lines Definition Chemistry When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. The reason the chemical elements can do this is. The lines that a particular element produces are called the emission spectrum of that element. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and.
From sciencedemos.org.uk
The Spectral Lines of the Hydrogen Atom Spectral Lines Definition Chemistry Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. The lines that a particular element produces are called the emission spectrum of that element. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range. Spectral Lines Definition Chemistry.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Spectral Lines Definition Chemistry The reason the chemical elements can do this is. Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. The lines that a particular element produces are called the emission. Spectral Lines Definition Chemistry.
From sankemta.blogspot.com
CHEMISTRY THEORY and REVISION Spectral Lines Definition Chemistry These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. The lines that a particular element produces are called the emission spectrum of that element. The reason the chemical elements can do this is. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements.. Spectral Lines Definition Chemistry.
From courses.lumenlearning.com
Spectroscopy in Astronomy Astronomy Spectral Lines Definition Chemistry Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. These lines are called. Spectral Lines Definition Chemistry.
From byjus.com
Spectral Series Explained along with Hydrogen spectrum, Rydberg formula. Spectral Lines Definition Chemistry The reason the chemical elements can do this is. Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range. Spectral Lines Definition Chemistry.
From education-portal.com
Electron Transitions & Spectral Lines Video & Lesson Transcript Spectral Lines Definition Chemistry The reason the chemical elements can do this is. Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range. Spectral Lines Definition Chemistry.
From www.youtube.com
Using Spectral Lines to Determine What Elements are in Stars YouTube Spectral Lines Definition Chemistry The lines that a particular element produces are called the emission spectrum of that element. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range. Spectral Lines Definition Chemistry.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Spectral Lines Definition Chemistry Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. The reason the chemical elements can do this is. A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called. Spectral Lines Definition Chemistry.
From classnotes.org.in
Hydrogen spectrum Chemistry, Class 11, Structure Of Atom Spectral Lines Definition Chemistry Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. When. Spectral Lines Definition Chemistry.
From byjus.com
Hydrogen Spectrum Balmer SeriesDefinitionDiagram Chemistry Byju’s Spectral Lines Definition Chemistry The lines that a particular element produces are called the emission spectrum of that element. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules.. Spectral Lines Definition Chemistry.
From studylib.net
Broadening of spectral lines An individual atom/molecule making a Spectral Lines Definition Chemistry The reason the chemical elements can do this is. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. Grasp the definition of spectroscopy and. Spectral Lines Definition Chemistry.
From wisc.pb.unizin.org
Day 2 Atomic Spectra and Atomic Orbitals Chemistry 109, Fall 2020 Spectral Lines Definition Chemistry Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. The lines that a particular element produces are called the emission spectrum of that element. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. A spectral line is a specific wavelength of light. Spectral Lines Definition Chemistry.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Spectral Lines Definition Chemistry Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. A spectral line is a specific wavelength of light emitted or absorbed by atoms. Spectral Lines Definition Chemistry.
From chem.libretexts.org
14.2 LineBroadening and Spectral Diffusion Chemistry LibreTexts Spectral Lines Definition Chemistry A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. The reason the chemical elements can do this is. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic. Spectral Lines Definition Chemistry.
From www.slideserve.com
PPT Chapter 3 Spectral lines in stars PowerPoint Presentation, free Spectral Lines Definition Chemistry When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. The reason the chemical elements can do this is. Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms. Spectral Lines Definition Chemistry.
From rightmetal.weebly.com
Atomic emission spectrum chemistry definition rightmetal Spectral Lines Definition Chemistry These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. The reason the chemical elements can do this is. A spectral line. Spectral Lines Definition Chemistry.
From studylib.net
Widths of spectral lines Spectral Lines Definition Chemistry Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line. Spectral Lines Definition Chemistry.
From studylib.net
SPECTRAL LINES OF THE ELEMENTS Spectral Lines Definition Chemistry The reason the chemical elements can do this is. A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. The lines that a particular element produces are called the emission spectrum of that element. These lines are. Spectral Lines Definition Chemistry.
From www.teachmint.com
Intensity Of Spectral Lines Chemistry Notes Teachmint Spectral Lines Definition Chemistry The lines that a particular element produces are called the emission spectrum of that element. Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. A spectral line. Spectral Lines Definition Chemistry.
From saylordotorg.github.io
Atomic Spectra and Models of the Atom Spectral Lines Definition Chemistry Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. The reason the chemical elements can do this is. A spectral line is a specific wavelength of light emitted or. Spectral Lines Definition Chemistry.
From chem.libretexts.org
6.3 Line Spectra and the Bohr Model Chemistry LibreTexts Spectral Lines Definition Chemistry These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. When. Spectral Lines Definition Chemistry.
From classnotes.org.in
Absorption and Emission Spectra Chemistry, Class 11, Structure of Atom Spectral Lines Definition Chemistry Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. When the. Spectral Lines Definition Chemistry.
From www.slideserve.com
PPT Big Bang PowerPoint Presentation, free download ID3406435 Spectral Lines Definition Chemistry When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. The lines that a particular element produces are called the emission spectrum of that element. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for. Spectral Lines Definition Chemistry.
From infinitylearn.com
Line spectrum contains information about Spectral Lines Definition Chemistry When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. The reason the chemical elements can do this is. Grasp the definition. Spectral Lines Definition Chemistry.
From www.mrpalermo.com
Bright Line Spectra Mr. Palermo's Flipped Chemistry Classroom Spectral Lines Definition Chemistry These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. The. Spectral Lines Definition Chemistry.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Spectral Lines Definition Chemistry A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. The lines that a particular element produces are called the emission spectrum of that element. The reason the chemical elements can do this is. Grasp. Spectral Lines Definition Chemistry.
From www.artofit.org
Chapter 7 spectral lines Artofit Spectral Lines Definition Chemistry Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. The reason the chemical elements can do this is. When the emitted light is passed through a prism, only a few narrow lines of. Spectral Lines Definition Chemistry.
From studylib.net
The intensity of spectral lines depends on Spectral Lines Definition Chemistry The reason the chemical elements can do this is. The lines that a particular element produces are called the emission spectrum of that element. A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. Grasp. Spectral Lines Definition Chemistry.
From socratic.org
Why are atomic spectra of an element discontinuous? Socratic Spectral Lines Definition Chemistry When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by. Spectral Lines Definition Chemistry.
From mavink.com
Visible Line Spectrum Of Hydrogen Spectral Lines Definition Chemistry The lines that a particular element produces are called the emission spectrum of that element. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. The reason the chemical elements can do this is. Spectral lines are distinct lines or bands. Spectral Lines Definition Chemistry.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Spectral Lines Definition Chemistry The lines that a particular element produces are called the emission spectrum of that element. Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. These lines are called spectra and. Spectral Lines Definition Chemistry.
From www.slideserve.com
PPT Spectral lines PowerPoint Presentation, free download ID301037 Spectral Lines Definition Chemistry Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. The lines that a particular element produces are called the emission spectrum of that element. Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. When the emitted light is. Spectral Lines Definition Chemistry.
From www.thoughtco.com
What Is Luminosity and What does it Tell Us? Spectral Lines Definition Chemistry The reason the chemical elements can do this is. When the emitted light is passed through a prism, only a few narrow lines of particular wavelengths, called a line spectrum, are observed rather than a continuous range of. Grasp the definition of spectroscopy and a spectrum as the most basic item reported by spectroscopic measurements. Spectral lines are distinct lines. Spectral Lines Definition Chemistry.
From sdsu-physics.org
Quantum Physics Spectral Lines Definition Chemistry Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is \(\lambda\)) for a specific element. The lines that a particular element produces are called the emission spectrum of that element. When the emitted. Spectral Lines Definition Chemistry.
From spiff.rit.edu
Spectrographs and Spectra Spectral Lines Definition Chemistry Spectral lines are distinct lines or bands in a spectrum that correspond to specific wavelengths of light emitted or absorbed by atoms and. A spectral line is a specific wavelength of light emitted or absorbed by atoms or molecules. The lines that a particular element produces are called the emission spectrum of that element. Grasp the definition of spectroscopy and. Spectral Lines Definition Chemistry.