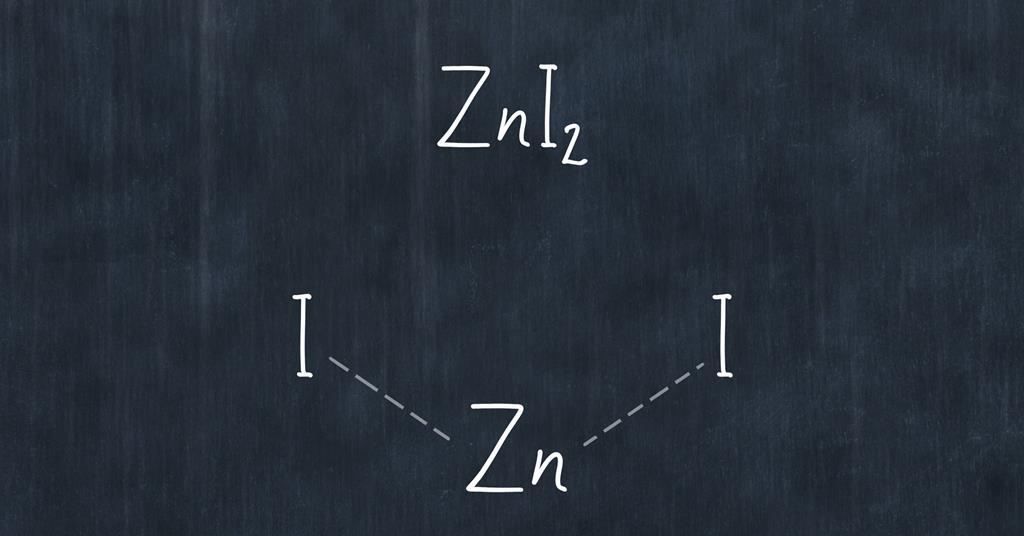
Zinc Iodide Reaction Equation . Diiodine + zinc = zinc iodide. This can be reversed using electrolysis to decompose the compound. Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. Zinc iodide is a white crystalline solid with the formula zni2. Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Zinc powder is added to a solution of iodine in ethanol. Zinc iodide = zinc + iodine. Use the algebraic method, the inspection method, or the. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. It decomposes when heated, reacts with strong bases, and is used as a reducing. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn].
from edu-rsc-org-ssl.oca.korea.ac.kr
Use the algebraic method, the inspection method, or the. Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. It decomposes when heated, reacts with strong bases, and is used as a reducing. Zinc iodide = zinc + iodine. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zinc powder is added to a solution of iodine in ethanol. Diiodine + zinc = zinc iodide. This can be reversed using electrolysis to decompose the compound. Zinc iodide is a white crystalline solid with the formula zni2.
Exothermic redox reaction of zinc with iodine Experiment RSC Education
Zinc Iodide Reaction Equation Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. Zinc iodide is a white crystalline solid with the formula zni2. This can be reversed using electrolysis to decompose the compound. Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. It decomposes when heated, reacts with strong bases, and is used as a reducing. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Use the algebraic method, the inspection method, or the. Diiodine + zinc = zinc iodide. Zinc powder is added to a solution of iodine in ethanol. Zinc iodide = zinc + iodine.
From www.toppr.com
Balanced equation for the reaction between zinc and dilute sulphuric Zinc Iodide Reaction Equation Zinc iodide is a white crystalline solid with the formula zni2. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. Zni2 = zn + i is a decomposition reaction where one mole. Zinc Iodide Reaction Equation.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc Iodide Reaction Equation I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zinc iodide is a white crystalline solid with the formula zni2. This can be reversed using electrolysis to decompose the compound. It decomposes when heated, reacts with strong bases, and is used as a reducing. Diiodine + zinc. Zinc Iodide Reaction Equation.
From www.youtube.com
How to Write the Formula for Zinc iodide YouTube Zinc Iodide Reaction Equation I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zinc iodide is a white crystalline solid with the formula zni2. Diiodine + zinc = zinc iodide. Use the algebraic method, the inspection method, or the. Zinc iodide = zinc + iodine. Learn how to balance the equation. Zinc Iodide Reaction Equation.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc Iodide Reaction Equation Zinc powder is added to a solution of iodine in ethanol. Zinc iodide is a white crystalline solid with the formula zni2. This can be reversed using electrolysis to decompose the compound. It decomposes when heated, reacts with strong bases, and is used as a reducing. Zinc iodide = zinc + iodine. I2 + zn = zni2 is a synthesis. Zinc Iodide Reaction Equation.
From www.nagwa.com
Question Video Identifying the Reason for Melting Zinc Iodide before Zinc Iodide Reaction Equation Use the algebraic method, the inspection method, or the. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zinc iodide is a white crystalline solid with the formula zni2. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2]. Zinc Iodide Reaction Equation.
From www.toppr.com
Identify the type of reactions taking place in each of the following Zinc Iodide Reaction Equation This can be reversed using electrolysis to decompose the compound. Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. It decomposes when heated, reacts with strong bases, and is used as a. Zinc Iodide Reaction Equation.
From www.numerade.com
SOLVED A Synthesis and formula of zinc iodide 1 Write the expected Zinc Iodide Reaction Equation Diiodine + zinc = zinc iodide. This can be reversed using electrolysis to decompose the compound. Use the algebraic method, the inspection method, or the. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. Zinc iodide = zinc + iodine. I2 + zn = zni2 is a synthesis reaction. Zinc Iodide Reaction Equation.
From edu-rsc-org-ssl.oca.korea.ac.kr
Exothermic redox reaction of zinc with iodine Experiment RSC Education Zinc Iodide Reaction Equation Diiodine + zinc = zinc iodide. Zinc iodide is a white crystalline solid with the formula zni2. It decomposes when heated, reacts with strong bases, and is used as a reducing. This can be reversed using electrolysis to decompose the compound. Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Zinc powder is added to. Zinc Iodide Reaction Equation.
From pubs.rsc.org
Zinc iodide a mild and efficient catalyst for onepot synthesis of Zinc Iodide Reaction Equation Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. It decomposes when heated, reacts with strong bases, and is used. Zinc Iodide Reaction Equation.
From www.nagwa.com
Question Video Identifying the Correct Chemical Equation for the Zinc Iodide Reaction Equation Zinc iodide = zinc + iodine. Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Zinc iodide is a white crystalline solid with the formula zni2. This can be reversed using electrolysis to decompose the compound. Zinc powder is added. Zinc Iodide Reaction Equation.
From www.chegg.com
Solved PRELAB QUESTION Balanced equations for the reactions Zinc Iodide Reaction Equation Zinc iodide is a white crystalline solid with the formula zni2. This can be reversed using electrolysis to decompose the compound. Diiodine + zinc = zinc iodide. Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole. Zinc Iodide Reaction Equation.
From studylib.net
Synthesis of Zinc Iodide Tracking a Chemical Reaction Zinc Iodide Reaction Equation Zinc powder is added to a solution of iodine in ethanol. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. This can be reversed using electrolysis to decompose the compound. Use the algebraic method, the inspection method, or the. Zinc iodide is a white crystalline solid with the formula. Zinc Iodide Reaction Equation.
From www.slideserve.com
PPT Reaction of Zinc and Iodine PowerPoint Presentation, free Zinc Iodide Reaction Equation It decomposes when heated, reacts with strong bases, and is used as a reducing. Zinc iodide is a white crystalline solid with the formula zni2. Diiodine + zinc = zinc iodide. Zinc iodide = zinc + iodine. Use the algebraic method, the inspection method, or the. Zinc powder is added to a solution of iodine in ethanol. I2 + zn. Zinc Iodide Reaction Equation.
From www.shutterstock.com
Zinc Iodide Zni2 Molecule Simple Molecular Stock Vector (Royalty Free Zinc Iodide Reaction Equation Zinc iodide is a white crystalline solid with the formula zni2. Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. This can be reversed using electrolysis to decompose the compound. Zni2 =. Zinc Iodide Reaction Equation.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc Iodide Reaction Equation Zinc iodide = zinc + iodine. Diiodine + zinc = zinc iodide. Zinc iodide is a white crystalline solid with the formula zni2. It decomposes when heated, reacts with strong bases, and is used as a reducing. Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Zni2 = zn + i is a decomposition reaction. Zinc Iodide Reaction Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + ZnSO4 = Zn + MgSO4 (See Zinc Iodide Reaction Equation Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. This can be reversed using electrolysis to decompose the compound. Zinc powder is added to a solution of iodine in ethanol. Use the algebraic method, the inspection method, or the. Zinc iodide = zinc + iodine. Learn how to balance the equation of zinc and iodine. Zinc Iodide Reaction Equation.
From www.toppr.com
In the reaction between zinc and iodine, zinc iodide is formed. Which Zinc Iodide Reaction Equation Zinc iodide is a white crystalline solid with the formula zni2. This can be reversed using electrolysis to decompose the compound. It decomposes when heated, reacts with strong bases, and is used as a reducing. Zinc powder is added to a solution of iodine in ethanol. Diiodine + zinc = zinc iodide. Using an exothermic redox reaction between zinc and. Zinc Iodide Reaction Equation.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc Iodide Reaction Equation I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Diiodine + zinc = zinc iodide. Zinc iodide = zinc + iodine. Zinc iodide is a white crystalline solid with the formula zni2. Learn how to balance the equation of zinc and iodine reacting to form zinc iodide.. Zinc Iodide Reaction Equation.
From www.youtube.com
Finding the Empirical Formula For Zinc Iodide General Chemistry Zinc Iodide Reaction Equation Use the algebraic method, the inspection method, or the. Zinc iodide is a white crystalline solid with the formula zni2. This can be reversed using electrolysis to decompose the compound. It decomposes when heated, reacts with strong bases, and is used as a reducing. Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. Diiodine. Zinc Iodide Reaction Equation.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc Iodide Reaction Equation Diiodine + zinc = zinc iodide. Zinc iodide is a white crystalline solid with the formula zni2. Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. This can be reversed using electrolysis to decompose the compound. Zinc iodide = zinc + iodine. Use the algebraic method, the inspection method, or the. Learn how to balance. Zinc Iodide Reaction Equation.
From www.youtube.com
How to Balance AgNO3 + ZnI2 = AgI + Zn(NO3)2 Silver Nitrate + Zinc Zinc Iodide Reaction Equation Zinc iodide = zinc + iodine. Use the algebraic method, the inspection method, or the. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zinc iodide is a white crystalline solid with the formula zni2. Using an exothermic redox reaction between zinc and iodine, student will make. Zinc Iodide Reaction Equation.
From www.chegg.com
Solved Balanced equations for the reactions occurring in the Zinc Iodide Reaction Equation Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Diiodine + zinc = zinc iodide. Zinc iodide = zinc + iodine. Zinc iodide is a white crystalline solid with the formula zni2. This can be reversed using electrolysis to decompose. Zinc Iodide Reaction Equation.
From www.toppr.com
In the reaction between zinc and iodine , zinc iodide is formed . What Zinc Iodide Reaction Equation Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. Zinc iodide is a white crystalline solid with the formula zni2. It decomposes when heated, reacts with strong bases, and is used as a reducing. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i. Zinc Iodide Reaction Equation.
From www.youtube.com
How to Balance H2SO4 + Zn = ZnSO4 + H2 (Sulfuric acid + Zinc) YouTube Zinc Iodide Reaction Equation I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Use the algebraic method, the inspection method, or the. Zinc iodide = zinc + iodine. Diiodine + zinc = zinc iodide. Zinc powder is added to a solution of iodine in ethanol. Zni2 = zn + i is. Zinc Iodide Reaction Equation.
From www.numerade.com
SOLVED Zinc reacts with iodine in a synthesis reaction. Using a Zinc Iodide Reaction Equation Zinc iodide = zinc + iodine. Zinc powder is added to a solution of iodine in ethanol. It decomposes when heated, reacts with strong bases, and is used as a reducing. Diiodine + zinc = zinc iodide. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zinc. Zinc Iodide Reaction Equation.
From www.numerade.com
SOLVED What is the expected empirical formula for the compound that Zinc Iodide Reaction Equation Zinc powder is added to a solution of iodine in ethanol. Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. This can be reversed using electrolysis to decompose the compound. Use the algebraic method, the inspection method, or the. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i. Zinc Iodide Reaction Equation.
From keplarllp.com
😍 What is the formula for zinc iodide. Formula for zinc iodide. 20190204 Zinc Iodide Reaction Equation Zinc iodide is a white crystalline solid with the formula zni2. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. It decomposes when heated, reacts with strong bases, and is used as a reducing. This can be reversed using electrolysis to decompose the compound. Zinc iodide =. Zinc Iodide Reaction Equation.
From www.slideserve.com
PPT Making zinc iodide from zinc and iodine PowerPoint Presentation Zinc Iodide Reaction Equation This can be reversed using electrolysis to decompose the compound. Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. It decomposes when heated, reacts with strong bases, and is used as a reducing. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn].. Zinc Iodide Reaction Equation.
From www.numerade.com
SOLVED PRELAB QUESTION Balanced equations for the reactions Zinc Iodide Reaction Equation Use the algebraic method, the inspection method, or the. Zinc iodide = zinc + iodine. Diiodine + zinc = zinc iodide. It decomposes when heated, reacts with strong bases, and is used as a reducing. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. Zinc powder is added to. Zinc Iodide Reaction Equation.
From www.researchgate.net
(PDF) Synthesis and of Zinc Iodide Model Reactions for Zinc Iodide Reaction Equation Zinc iodide is a white crystalline solid with the formula zni2. Diiodine + zinc = zinc iodide. Use the algebraic method, the inspection method, or the. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. Learn how to balance the equation of zinc and iodine reacting to form zinc. Zinc Iodide Reaction Equation.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Zinc Iodide Reaction Equation Zinc iodide = zinc + iodine. This can be reversed using electrolysis to decompose the compound. It decomposes when heated, reacts with strong bases, and is used as a reducing. Diiodine + zinc = zinc iodide. Zinc powder is added to a solution of iodine in ethanol. Using an exothermic redox reaction between zinc and iodine, student will make zinc. Zinc Iodide Reaction Equation.
From www.pw.live
Zinc Iodide Formula, Structure, Properties And Uses Zinc Iodide Reaction Equation I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zinc iodide is a white crystalline solid with the formula zni2. Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. Diiodine + zinc = zinc iodide. Zni2 = zn + i is. Zinc Iodide Reaction Equation.
From slideplayer.com
Classifying Chemical Reactions Types ppt download Zinc Iodide Reaction Equation Learn how to balance the equation of zinc and iodine reacting to form zinc iodide. Zinc powder is added to a solution of iodine in ethanol. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zni2 = zn + i is a decomposition reaction where one mole. Zinc Iodide Reaction Equation.
From www.slideserve.com
PPT Unit 1 Review Quantitative Chemistry PowerPoint Presentation Zinc Iodide Reaction Equation Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. I2 + zn = zni2 is a synthesis reaction where one mole of diiodine [i 2] and one mole of zinc [zn]. Zinc powder is added to a solution of iodine in ethanol. This can be reversed using electrolysis to decompose the compound. Use the algebraic. Zinc Iodide Reaction Equation.
From pubs.acs.org
Zinc Iodide Catalyzed Synthesis of Trisubstituted Allenes from Terminal Zinc Iodide Reaction Equation Zinc iodide = zinc + iodine. Zinc powder is added to a solution of iodine in ethanol. This can be reversed using electrolysis to decompose the compound. It decomposes when heated, reacts with strong bases, and is used as a reducing. Use the algebraic method, the inspection method, or the. Diiodine + zinc = zinc iodide. Zni2 = zn +. Zinc Iodide Reaction Equation.