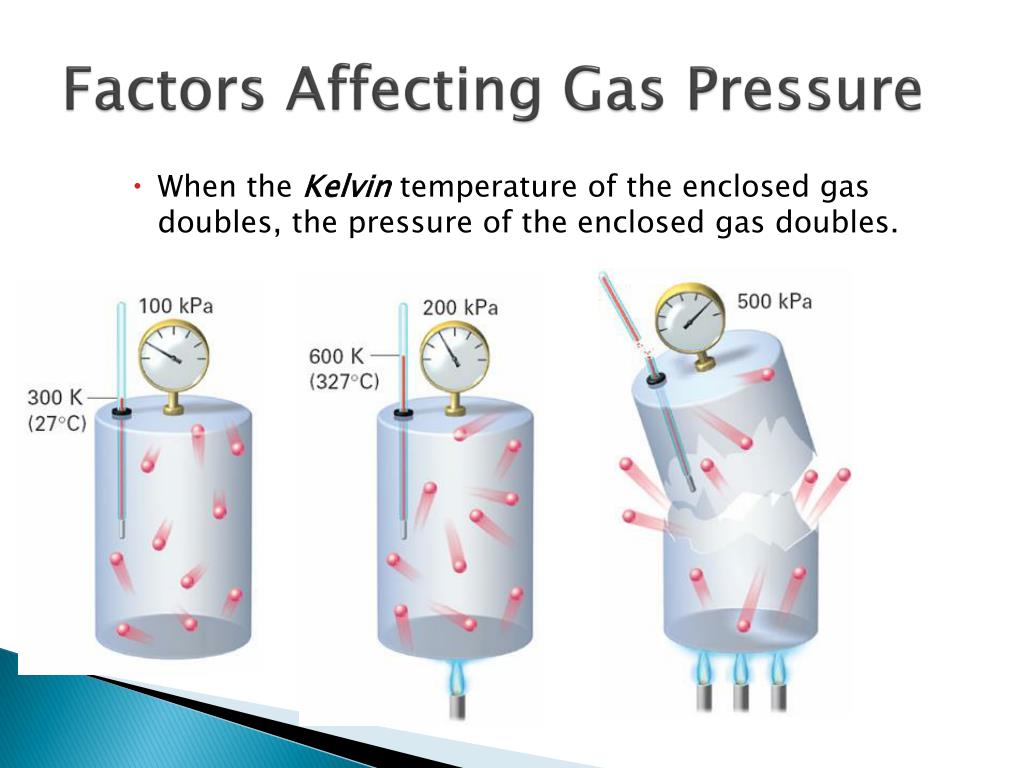
What Three Factors Affect Gas Pressure . gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. Pressure in gases is caused by particles. With the walls of the container. At the macroscopic level, a complete physical description of a sample of a gas. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). to describe and measure the pressure of a gas. factors affecting gas pressure. The pressure of the air in a basketball has to be adjusted so that the. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. Gas pressure is increased when.
from www.slideserve.com
they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. factors affecting gas pressure. Pressure in gases is caused by particles. At the macroscopic level, a complete physical description of a sample of a gas. The pressure of the air in a basketball has to be adjusted so that the. to describe and measure the pressure of a gas. With the walls of the container. Gas pressure is increased when. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1).
PPT The Behavior of Gases PowerPoint Presentation, free download ID
What Three Factors Affect Gas Pressure gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. Pressure in gases is caused by particles. The pressure of the air in a basketball has to be adjusted so that the. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. factors affecting gas pressure. With the walls of the container. to describe and measure the pressure of a gas. At the macroscopic level, a complete physical description of a sample of a gas. Gas pressure is increased when.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID6583940 What Three Factors Affect Gas Pressure gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). Pressure in gases is caused by particles. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. The pressure of the air in a basketball has to be adjusted so. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Chapter 14 The Behavior of Gases PowerPoint Presentation, free What Three Factors Affect Gas Pressure to describe and measure the pressure of a gas. With the walls of the container. The pressure of the air in a basketball has to be adjusted so that the. factors affecting gas pressure. At the macroscopic level, a complete physical description of a sample of a gas. Pressure in gases is caused by particles. gas pressure. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Unit 13 PowerPoint Presentation, free download ID2214586 What Three Factors Affect Gas Pressure they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. With the walls of the container. to describe and measure the pressure of a gas. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). Gas pressure is. What Three Factors Affect Gas Pressure.
From www.youtube.com
Factors Affecting Gas Pressure YouTube What Three Factors Affect Gas Pressure factors affecting gas pressure. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. The pressure of the air in a basketball has to be adjusted so that. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT The Behavior of Gases PowerPoint Presentation, free download ID What Three Factors Affect Gas Pressure gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). The pressure of the air in a basketball has to be adjusted so that the. Pressure in gases is caused by particles. to describe and measure the pressure of a gas. factors affecting gas pressure. At the macroscopic. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Chemistry 14.1 PowerPoint Presentation, free download ID4874125 What Three Factors Affect Gas Pressure With the walls of the container. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. to describe and measure the pressure of a gas. Pressure in gases is caused by particles. Gas pressure is increased when. The pressure of the air in a basketball has to be. What Three Factors Affect Gas Pressure.
From slidetodoc.com
Chapter 14 The Behavior of Gases Properties of What Three Factors Affect Gas Pressure they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. to describe and measure the pressure of a gas. Gas pressure is increased when. At the macroscopic level,. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Chapter 14 The Behavior of Gases PowerPoint Presentation, free What Three Factors Affect Gas Pressure Gas pressure is increased when. Pressure in gases is caused by particles. to describe and measure the pressure of a gas. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by. What Three Factors Affect Gas Pressure.
From www.scribd.com
Chapter 122 Factors Affecting Gas Pressure PDF What Three Factors Affect Gas Pressure factors affecting gas pressure. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). Pressure in gases is caused by particles. At the macroscopic level, a complete physical description of a sample of a gas. they are pressure (p), volume (v), temperature (t), and the amount of the. What Three Factors Affect Gas Pressure.
From slideplayer.com
The Behavior of Gases. ppt download What Three Factors Affect Gas Pressure to describe and measure the pressure of a gas. The pressure of the air in a basketball has to be adjusted so that the. Pressure in gases is caused by particles. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. Gas pressure is increased when. With the. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Chemistry 14.1 PowerPoint Presentation, free download ID1019258 What Three Factors Affect Gas Pressure factors affecting gas pressure. to describe and measure the pressure of a gas. At the macroscopic level, a complete physical description of a sample of a gas. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. they are pressure (p), volume (v), temperature (t), and the. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Chapter 14 The Behavior of Gases PowerPoint Presentation, free What Three Factors Affect Gas Pressure The pressure of the air in a basketball has to be adjusted so that the. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. gas pressure is. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Factors that Affect Gas Pressure PowerPoint Presentation, free What Three Factors Affect Gas Pressure they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Chapter 14 The Behavior of Gases PowerPoint Presentation, free What Three Factors Affect Gas Pressure At the macroscopic level, a complete physical description of a sample of a gas. Gas pressure is increased when. The pressure of the air in a basketball has to be adjusted so that the. Pressure in gases is caused by particles. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the. What Three Factors Affect Gas Pressure.
From slideplayer.com
Chapter 14 The Behavior of Gases 14.1 Properties of Gases ppt download What Three Factors Affect Gas Pressure gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. Pressure in gases is caused by particles. At the macroscopic level, a complete physical description of a sample of. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT 3.2 The Gas Laws PowerPoint Presentation, free download ID6400527 What Three Factors Affect Gas Pressure Pressure in gases is caused by particles. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. to describe and measure the pressure of a gas. factors. What Three Factors Affect Gas Pressure.
From www.youtube.com
Calculating Gas Pressure Pressure in the Gas Laws CLEAR & SIMPLE What Three Factors Affect Gas Pressure Gas pressure is increased when. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. to describe and measure the pressure of a gas. Pressure in gases is caused by particles. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT To learn about atmospheric pressure and how barometers work To What Three Factors Affect Gas Pressure factors affecting gas pressure. With the walls of the container. to describe and measure the pressure of a gas. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. At the macroscopic level, a complete physical description of a sample of a gas. The pressure of the. What Three Factors Affect Gas Pressure.
From slideplayer.com
Chapter 14 The Behavior of Gases 14.1 Properties of Gases ppt download What Three Factors Affect Gas Pressure Gas pressure is increased when. At the macroscopic level, a complete physical description of a sample of a gas. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles.. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT CHEMISTRY PowerPoint Presentation, free download ID4186573 What Three Factors Affect Gas Pressure they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. With the walls of the container. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). Gas pressure is increased when. Pressure in gases is caused by particles. . What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Compressibility PowerPoint Presentation, free download ID9567804 What Three Factors Affect Gas Pressure Gas pressure is increased when. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. At the macroscopic level, a complete physical description of a sample of a gas. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1).. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Ch. 12 Behavior of Gases PowerPoint Presentation, free download What Three Factors Affect Gas Pressure The pressure of the air in a basketball has to be adjusted so that the. With the walls of the container. Gas pressure is increased when. Pressure in gases is caused by particles. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. to describe and measure the pressure. What Three Factors Affect Gas Pressure.
From slideplayer.com
CHAPTER 14 THE BEHAVIOR OF GASES ppt download What Three Factors Affect Gas Pressure Gas pressure is increased when. The pressure of the air in a basketball has to be adjusted so that the. At the macroscopic level, a complete physical description of a sample of a gas. factors affecting gas pressure. With the walls of the container. gas pressure in a closed container is the result of the gas molecules hitting. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID2829261 What Three Factors Affect Gas Pressure The pressure of the air in a basketball has to be adjusted so that the. With the walls of the container. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. factors affecting gas pressure. At the macroscopic level, a complete physical description of a sample of a gas.. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Factors that Affect Gas Pressure PowerPoint Presentation, free What Three Factors Affect Gas Pressure gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. With the walls of the container. factors affecting gas pressure. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. Pressure in gases is caused by particles. Gas. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Chemistry 14.1 PowerPoint Presentation, free download ID4874125 What Three Factors Affect Gas Pressure Pressure in gases is caused by particles. Gas pressure is increased when. to describe and measure the pressure of a gas. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). gas pressure in a closed container is the result of the gas molecules hitting the inside of. What Three Factors Affect Gas Pressure.
From slideplayer.com
Chemistry Trimester 2 Jeopardy ppt download What Three Factors Affect Gas Pressure gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. At the macroscopic level, a complete physical description of a sample of a gas. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. With the walls of the. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID1414769 What Three Factors Affect Gas Pressure gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). Pressure in gases is caused by particles. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. they are pressure (p), volume (v), temperature (t), and the amount of. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT The Behavior of Gases PowerPoint Presentation, free download ID What Three Factors Affect Gas Pressure At the macroscopic level, a complete physical description of a sample of a gas. Pressure in gases is caused by particles. to describe and measure the pressure of a gas. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. factors affecting gas pressure. Gas pressure is increased. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Chapter 14 The Behavior of Gases PowerPoint Presentation, free What Three Factors Affect Gas Pressure Pressure in gases is caused by particles. factors affecting gas pressure. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). At the macroscopic level, a complete physical description of a sample of a gas. gas pressure in a closed container is the result of the gas molecules. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Chapter 3 States of Matter PowerPoint Presentation, free download What Three Factors Affect Gas Pressure factors affecting gas pressure. Gas pressure is increased when. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. The pressure of the air in a basketball has to be adjusted so that the. At the macroscopic level, a complete physical description of a sample of a gas. With. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT Chemistry 14.1 PowerPoint Presentation, free download ID996052 What Three Factors Affect Gas Pressure The pressure of the air in a basketball has to be adjusted so that the. gas pressure in a closed container is the result of the gas molecules hitting the inside of the container. factors affecting gas pressure. With the walls of the container. At the macroscopic level, a complete physical description of a sample of a gas.. What Three Factors Affect Gas Pressure.
From slideplayer.com
Chapter 14 The Behavior of Gases ppt download What Three Factors Affect Gas Pressure Gas pressure is increased when. At the macroscopic level, a complete physical description of a sample of a gas. to describe and measure the pressure of a gas. they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. Pressure in gases is caused by particles. The pressure of. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT States of Matter & Phase Changes PowerPoint Presentation ID6286577 What Three Factors Affect Gas Pressure they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number moles. With the walls of the container. factors affecting gas pressure. The pressure of the air in a basketball has to be adjusted so that the. At the macroscopic level, a complete physical description of a sample of a. What Three Factors Affect Gas Pressure.
From www.slideserve.com
PPT List and define the three states of matter. PowerPoint What Three Factors Affect Gas Pressure At the macroscopic level, a complete physical description of a sample of a gas. With the walls of the container. gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (figure 8.2.1). they are pressure (p), volume (v), temperature (t), and the amount of the gas as measured by the number. What Three Factors Affect Gas Pressure.