How Much Energy Required To Boil Water . That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. It surprised me that the energy required to warm up water doesn’t change with different starting. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o. To figure out the energy needed. At 1 atm, water freezes at 0° c and boils at 100° c. Phase changes in pure water occur at a specific temperature. The energy required to change water from a solid to a liquid is. This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c).
from www.chegg.com
I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. Phase changes in pure water occur at a specific temperature. The energy required to change water from a solid to a liquid is. The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). At 1 atm, water freezes at 0° c and boils at 100° c. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o. That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. To figure out the energy needed. It surprised me that the energy required to warm up water doesn’t change with different starting.
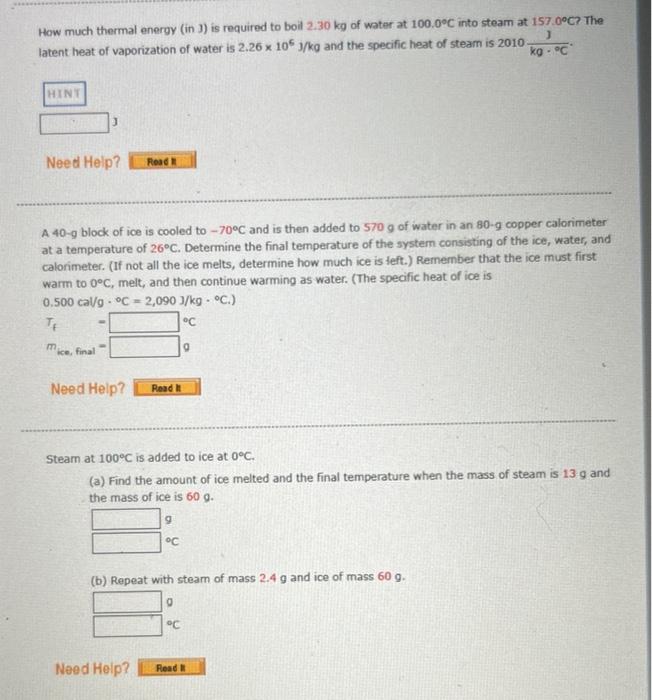
Solved How much thermal energy (in 3 ) is required to boil
How Much Energy Required To Boil Water At 1 atm, water freezes at 0° c and boils at 100° c. It surprised me that the energy required to warm up water doesn’t change with different starting. This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. At 1 atm, water freezes at 0° c and boils at 100° c. That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. Phase changes in pure water occur at a specific temperature. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o. The energy required to change water from a solid to a liquid is. The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). To figure out the energy needed.
From www.slideserve.com
PPT Heat of Fusion PowerPoint Presentation, free download ID2249917 How Much Energy Required To Boil Water The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o. At 1 atm, water freezes at 0° c and boils at 100° c. I am trying to. How Much Energy Required To Boil Water.
From www.youtube.com
5 Energy Required to Vaporize Water YouTube How Much Energy Required To Boil Water This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. It surprised me that the energy required to warm up water doesn’t change with different starting. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o. At 1. How Much Energy Required To Boil Water.
From www.chegg.com
Solved 9.4 How much energy is required to boil 500 g of room How Much Energy Required To Boil Water Phase changes in pure water occur at a specific temperature. This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. The energy required to change water from a solid to a liquid is. At 1 atm, water freezes at 0° c and boils at 100° c. I am trying to calculate. How Much Energy Required To Boil Water.
From www.vrogue.co
Why Does Water Vapor Form When Water Is Heated To Its vrogue.co How Much Energy Required To Boil Water This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. It surprised me that the energy required to warm up water doesn’t change with different starting. The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). At 1 atm, water freezes. How Much Energy Required To Boil Water.
From brainly.com
How much thermal energy is needed to boil 2.65 kg of water at its How Much Energy Required To Boil Water The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h. How Much Energy Required To Boil Water.
From hxenwthnj.blob.core.windows.net
How Much Energy Needed To Boil Water at William Cain blog How Much Energy Required To Boil Water This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. At 1 atm, water freezes at 0° c and boils at 100° c. That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. It surprised me that the energy required to warm up. How Much Energy Required To Boil Water.
From www.slideserve.com
PPT L 19 Thermodynamics [4] PowerPoint Presentation, free download How Much Energy Required To Boil Water That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. Phase changes in pure water occur at a specific temperature. The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). It surprised me that the energy required to warm up water doesn’t. How Much Energy Required To Boil Water.
From haipernews.com
How To Calculate Heat Capacity Of Water Haiper How Much Energy Required To Boil Water At 1 atm, water freezes at 0° c and boils at 100° c. It surprised me that the energy required to warm up water doesn’t change with different starting. Phase changes in pure water occur at a specific temperature. That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. The calculators use. How Much Energy Required To Boil Water.
From www.slideshare.net
Unit 1 Presentation How Much Energy Required To Boil Water To figure out the energy needed. The energy required to change water from a solid to a liquid is. The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). Phase changes in pure water occur at a specific temperature. At 1 atm, water freezes at 0° c and boils at. How Much Energy Required To Boil Water.
From doomsdayden.com
How To Boil Water Without Electricity In 6 Easy Steps How Much Energy Required To Boil Water I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. At 1 atm, water freezes at 0° c and boils at 100° c. The energy required to change water from a solid to a liquid is. To figure out the energy. How Much Energy Required To Boil Water.
From www.numerade.com
SOLVED The amount of heat energy needed to heat 200 g of water from 15 How Much Energy Required To Boil Water The energy required to change water from a solid to a liquid is. This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). To figure out the energy needed. At 1. How Much Energy Required To Boil Water.
From www.researchgate.net
Energy released by LPG in boiling 1500 mL water Download Scientific How Much Energy Required To Boil Water That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. To figure out the energy needed. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o. It surprised me that the energy required to warm up water doesn’t change. How Much Energy Required To Boil Water.
From www.quora.com
How much energy is required to raise the temperature of water from How Much Energy Required To Boil Water At 1 atm, water freezes at 0° c and boils at 100° c. I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. The calculators use a simple constant for how much energy is needed to heat water by 1 degree. How Much Energy Required To Boil Water.
From www.slideserve.com
PPT 2. How much heat is required to boil 7.3g of Ammonia? (NH 3 ) ( Δ How Much Energy Required To Boil Water The energy required to change water from a solid to a liquid is. That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). At 1 atm, water freezes at 0° c and. How Much Energy Required To Boil Water.
From www.youtube.com
How to Boil Water • Animated Infographic YouTube How Much Energy Required To Boil Water At 1 atm, water freezes at 0° c and boils at 100° c. I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. To figure out the energy needed. The calculators use a simple constant for how much energy is needed. How Much Energy Required To Boil Water.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii How Much Energy Required To Boil Water The energy required to change water from a solid to a liquid is. To figure out the energy needed. The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). At 1 atm, water freezes at 0° c and boils at 100° c. I am trying to calculate the amount of. How Much Energy Required To Boil Water.
From www.chegg.com
Solved Question1 1 pts Consider a 100 L water heater at 25C. How Much Energy Required To Boil Water I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o. The calculators use a simple constant for how. How Much Energy Required To Boil Water.
From www.chegg.com
Solved How much thermal energy (in 3 ) is required to boil How Much Energy Required To Boil Water I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. It surprised me that the energy required to warm up water doesn’t change with different starting. The energy required to change water from a solid to a liquid is. That makes. How Much Energy Required To Boil Water.
From www.slideserve.com
PPT Heat of Fusion PowerPoint Presentation, free download ID2249917 How Much Energy Required To Boil Water The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). Phase changes in pure water occur at a specific temperature. That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. Our water heating calculator can help you determine both the amount of. How Much Energy Required To Boil Water.
From brainly.com
How much energy does it take to boil 100 mL of water? How Much Energy Required To Boil Water The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). To figure out the energy needed. It surprised me that the energy required to warm up water doesn’t change with different starting. At 1 atm, water freezes at 0° c and boils at 100° c. That makes it roughly 0.116kwh. How Much Energy Required To Boil Water.
From mireya-blogmiller.blogspot.com
Energy Required to Boil 1 Litre of Water How Much Energy Required To Boil Water I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. At 1 atm, water freezes at 0° c and boils at 100° c. Our water heating calculator can help you determine both the amount of heat required to raise the temperature. How Much Energy Required To Boil Water.
From www.numerade.com
The heat of vaporization of a liquid (ΔHvap ) is the energy required to How Much Energy Required To Boil Water Phase changes in pure water occur at a specific temperature. I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). At. How Much Energy Required To Boil Water.
From www.numerade.com
SOLVED kettle containing 7kg of water has just reached it boiling How Much Energy Required To Boil Water Phase changes in pure water occur at a specific temperature. The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). It surprised me that the energy required to warm up water doesn’t change with different starting. The energy required to change water from a solid to a liquid is. At. How Much Energy Required To Boil Water.
From www.chegg.com
Solved Calculate the energy required to boil 100 ml of water How Much Energy Required To Boil Water That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. At 1 atm, water freezes at 0° c and boils at 100° c.. How Much Energy Required To Boil Water.
From www.youtube.com
Thermochemistry Water Phase Change Heat Calculation.wmv YouTube How Much Energy Required To Boil Water It surprised me that the energy required to warm up water doesn’t change with different starting. Phase changes in pure water occur at a specific temperature. The energy required to change water from a solid to a liquid is. To figure out the energy needed. That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of. How Much Energy Required To Boil Water.
From www.slideshare.net
Energy ch 16 How Much Energy Required To Boil Water The energy required to change water from a solid to a liquid is. Phase changes in pure water occur at a specific temperature. That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. I am trying to calculate the amount of energy required to bring 1 litre of water up to boil. How Much Energy Required To Boil Water.
From slideplayer.com
Quick Review What is energy? How is it measured? ppt download How Much Energy Required To Boil Water Phase changes in pure water occur at a specific temperature. This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o. The energy required to change water from a. How Much Energy Required To Boil Water.
From www.energy.gov
Infographic How does a boiling water reactor work? Department of Energy How Much Energy Required To Boil Water It surprised me that the energy required to warm up water doesn’t change with different starting. This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. The energy required to change water from a solid to a liquid is. The calculators use a simple constant for how much energy is needed. How Much Energy Required To Boil Water.
From poe.com
What is the amount of energy required to boil water? How much energy is How Much Energy Required To Boil Water That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. Phase changes in pure water occur at a specific temperature. At 1 atm, water freezes at 0° c and boils at 100° c. The energy required to change water from a solid to a liquid is. It surprised me that the energy. How Much Energy Required To Boil Water.
From hxenwthnj.blob.core.windows.net
How Much Energy Needed To Boil Water at William Cain blog How Much Energy Required To Boil Water Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o. That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. Phase changes in pure water occur at a specific temperature. This water heating calculator finds the amount of energy. How Much Energy Required To Boil Water.
From www.gauthmath.com
Solved The specific latent heat of vaporisation of water is 2,300,000 How Much Energy Required To Boil Water Phase changes in pure water occur at a specific temperature. I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. Our water. How Much Energy Required To Boil Water.
From moreref.com
How much energy does it take to raise the temperature of water? More REF How Much Energy Required To Boil Water The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). That makes it roughly 0.116kwh to boil a litre of water at 80% efficiency of the induction stove. It surprised me that the energy required to warm up water doesn’t change with different starting. Phase changes in pure water occur. How Much Energy Required To Boil Water.
From www.chegg.com
Solved For water, H2O, the heat of vaporization at its How Much Energy Required To Boil Water This water heating calculator finds the amount of energy and time required to cause a specific water temperature change. I am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c) and to. Phase changes in pure water occur at a specific temperature. To figure. How Much Energy Required To Boil Water.
From www.youtube.com
BOIL water. How much energy? cost? YouTube How Much Energy Required To Boil Water The energy required to change water from a solid to a liquid is. At 1 atm, water freezes at 0° c and boils at 100° c. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o. To figure out the energy needed. I am trying to calculate. How Much Energy Required To Boil Water.
From slideplayer.com
Calculations associated with Heating and Cooling Curves ppt download How Much Energy Required To Boil Water The calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). To figure out the energy needed. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o. This water heating calculator finds the amount of energy and time. How Much Energy Required To Boil Water.