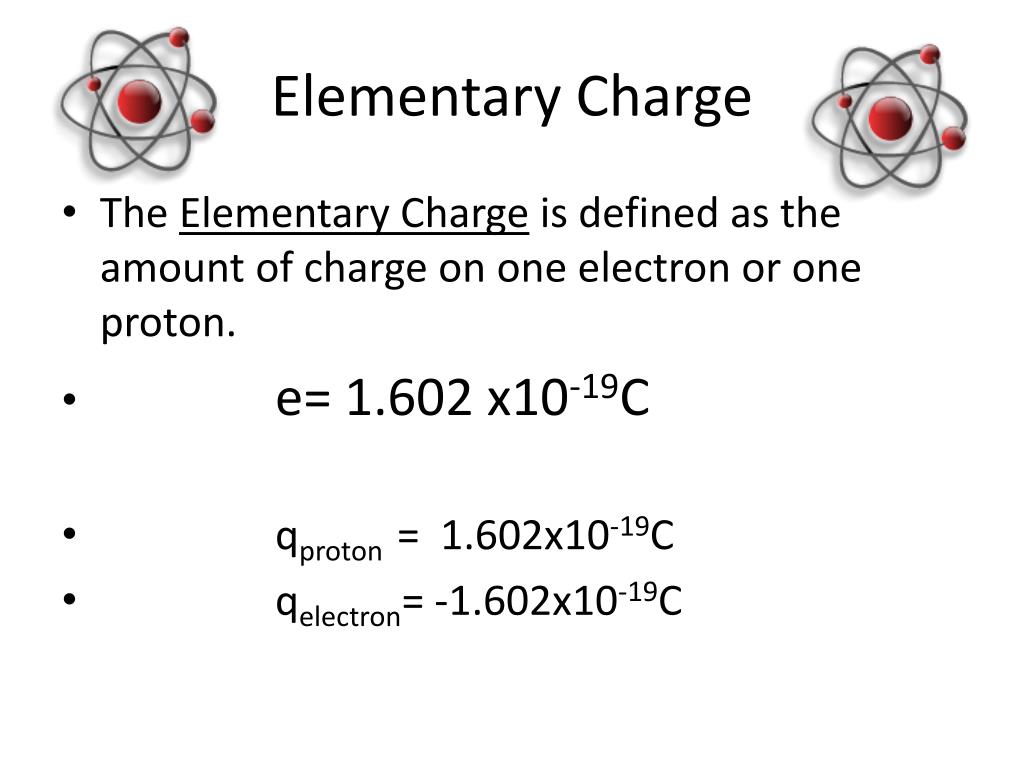
How To Find Charge Of Proton . Describe the locations, charges, and masses of the three main subatomic particles. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). To calculate the specific charge of a nucleus: Together with neutrons, they make up virtually all of the mass of an atom. Relative charges of −1 and +1 are assigned to the electron and proton,. Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): Determine the number of protons and electrons. Charges can be positive or negative,. A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and opposite to the negative charge. Specific charge (c/kg) = charge (c). Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton.
from sherysense.weebly.com
Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Specific charge (c/kg) = charge (c). Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). To calculate the specific charge of a nucleus: A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and opposite to the negative charge. Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): Charges can be positive or negative,. Relative charges of −1 and +1 are assigned to the electron and proton,. Together with neutrons, they make up virtually all of the mass of an atom.
A proton charge sherysense
How To Find Charge Of Proton Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and opposite to the negative charge. Relative charges of −1 and +1 are assigned to the electron and proton,. Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). To calculate the specific charge of a nucleus: Charges can be positive or negative,. Specific charge (c/kg) = charge (c). Together with neutrons, they make up virtually all of the mass of an atom. Describe the locations, charges, and masses of the three main subatomic particles. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): Determine the number of protons and electrons.
From byjus.com
Calculate the specific charges (e/m) of the following particles and How To Find Charge Of Proton Charges can be positive or negative,. Determine the number of protons and electrons. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Describe the locations, charges, and masses of the three main subatomic particles. A. How To Find Charge Of Proton.
From electricallive.com
Atomic Structure And Electric Charge Electrical engineering interview How To Find Charge Of Proton To calculate the specific charge of a nucleus: Together with neutrons, they make up virtually all of the mass of an atom. Relative charges of −1 and +1 are assigned to the electron and proton,. Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Charges can be positive or negative,.. How To Find Charge Of Proton.
From www.youtube.com
How to Determine Number of Protons, Neutrons, and Electrons. Step by How To Find Charge Of Proton Together with neutrons, they make up virtually all of the mass of an atom. Relative charges of −1 and +1 are assigned to the electron and proton,. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and opposite to. How To Find Charge Of Proton.
From www.thesciencehive.co.uk
Atomic Structure* — the science sauce How To Find Charge Of Proton A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and opposite to the negative charge. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Together with neutrons, they make up virtually all of the mass of an atom. Determine the number of protons and electrons. Specific charge (c/kg). How To Find Charge Of Proton.
From www.slideserve.com
PPT The Atom PowerPoint Presentation, free download ID5056341 How To Find Charge Of Proton Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. To calculate the specific charge of a nucleus: Together with neutrons, they make up virtually all of the mass of an atom. Charge is measured. How To Find Charge Of Proton.
From kamidd.best
How To Calculate The Number of Protons, Neutrons, and Electrons How To Find Charge Of Proton Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Together with neutrons, they make up virtually all of the mass of an atom. Relative charges of −1 and +1 are assigned to the electron and proton,. Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms):. How To Find Charge Of Proton.
From fabalabse.com
What are the three types of charges? Leia aqui What are the 3 types of How To Find Charge Of Proton Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Determine the number of protons and electrons. A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and. How To Find Charge Of Proton.
From www.youtube.com
Field of a Moving Charge, Proton, Right Hand Rule Physics How To Find Charge Of Proton Together with neutrons, they make up virtually all of the mass of an atom. Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Specific charge (c/kg) = charge (c). Determine the number of protons and electrons. Describe the locations, charges, and masses of the three main subatomic particles. To calculate. How To Find Charge Of Proton.
From www.youtube.com
P1 2 specific charge YouTube How To Find Charge Of Proton Together with neutrons, they make up virtually all of the mass of an atom. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and opposite to the negative charge. Describe the locations, charges, and masses of the three main. How To Find Charge Of Proton.
From period-faqs.com
How To Find Protons On Periodic Table How To Find Charge Of Proton Determine the number of protons and electrons. Together with neutrons, they make up virtually all of the mass of an atom. Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu).. How To Find Charge Of Proton.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Silver (Ag How To Find Charge Of Proton Relative charges of −1 and +1 are assigned to the electron and proton,. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. To calculate the specific charge of a nucleus: Describe the locations, charges, and masses of the three main subatomic particles. Specific charge is calculated by dividing the charge of a particle (in. How To Find Charge Of Proton.
From www.eeemadeeasy.com
Mass Of Electron, Proton, NeutronCharge Of Electron And Proton EEE How To Find Charge Of Proton Determine the number of protons and electrons. Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): Charges can be positive or negative,. Together with neutrons, they make up virtually all of the mass of an atom. Specific charge (c/kg) = charge (c). Relative charges of −1 and +1 are assigned to. How To Find Charge Of Proton.
From www.showme.com
Protons Neutrons and Electrons in S34 with 2 charge Science How To Find Charge Of Proton A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and opposite to the negative charge. Relative charges of −1 and +1 are assigned to the electron and proton,. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). Together with neutrons, they make up. How To Find Charge Of Proton.
From studyqueries.com
Charge Of Proton Definition, Mass & More How To Find Charge Of Proton Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): Charges can be positive or negative,. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Determine the. How To Find Charge Of Proton.
From studyqueries.com
Charge Of Proton Definition, Mass & More How To Find Charge Of Proton Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Charges can be positive or negative,. A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and opposite to the negative charge. Determine the number of protons and electrons. To calculate the specific charge of a. How To Find Charge Of Proton.
From ar.inspiredpencil.com
Proton Particle Charge How To Find Charge Of Proton Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). Together with neutrons, they make up virtually all of the mass of an atom. Determine the number of protons and electrons. Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): Charges. How To Find Charge Of Proton.
From www.ehow.com
How to Calculate the Charge of an Ion Sciencing How To Find Charge Of Proton Relative charges of −1 and +1 are assigned to the electron and proton,. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Specific charge (c/kg) = charge (c). Together with neutrons, they make up virtually all of the mass of an atom. Specific charge is calculated by dividing the charge of a particle (in. How To Find Charge Of Proton.
From www.youtube.com
3.4 Subatomic Particles Mass and Charge YouTube How To Find Charge Of Proton Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Specific charge (c/kg) = charge (c). A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and opposite to the negative charge. To calculate the specific charge of a nucleus: Determine the number of protons and. How To Find Charge Of Proton.
From www.breakingatom.com
Nuclear Charge How To Find Charge Of Proton Describe the locations, charges, and masses of the three main subatomic particles. Specific charge (c/kg) = charge (c). To calculate the specific charge of a nucleus: Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): Determine the number of protons and electrons. Protons have a positive electrical charge of one (1+). How To Find Charge Of Proton.
From www.youtube.com
Calculating charge using Electrons and Protons YouTube How To Find Charge Of Proton Charges can be positive or negative,. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. To calculate the specific charge of a nucleus: Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). Charge is measured in coulombs (c), which represent 6.242×10 18 e,. How To Find Charge Of Proton.
From www.youtube.com
How To Calculate The Number of Protons, Neutrons, and Electrons How To Find Charge Of Proton Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons. Together with neutrons, they make up virtually all of the mass of an atom. A proton is a fundamental subatomic particle with a. How To Find Charge Of Proton.
From www.tpsearchtool.com
3 Estructura Atomica Proton Elementos Quimicos Images How To Find Charge Of Proton Charges can be positive or negative,. Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): Describe the locations, charges, and masses of the three main subatomic particles. Relative charges of −1 and +1 are assigned to the electron and proton,. Together with neutrons, they make up virtually all of the mass. How To Find Charge Of Proton.
From www.numerade.com
SOLVED How do I find the over all charge of protons? How To Find Charge Of Proton Together with neutrons, they make up virtually all of the mass of an atom. Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): To calculate the specific charge of a nucleus: Relative charges of −1 and +1 are assigned to the electron and proton,. Charges can be positive or negative,. Electrons. How To Find Charge Of Proton.
From www.youtube.com
charge to mass ratio of a proton and electron YouTube How To Find Charge Of Proton To calculate the specific charge of a nucleus: Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Relative charges of −1 and +1 are assigned to the electron and proton,. Determine the number of protons and electrons. Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of. How To Find Charge Of Proton.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint How To Find Charge Of Proton Charges can be positive or negative,. Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Together with neutrons, they make up virtually all of the mass of an atom. A proton is a fundamental subatomic particle. How To Find Charge Of Proton.
From www.youtube.com
A proton is released from rest in a uniform electric field of magnitude How To Find Charge Of Proton Determine the number of protons and electrons. A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and opposite to the negative charge. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Charges can be positive or negative,. Charge is measured in coulombs (c), which represent 6.242×10 18 e,. How To Find Charge Of Proton.
From conquerchemistry.com
Lessons Archives Conquer Chemistry How To Find Charge Of Proton Together with neutrons, they make up virtually all of the mass of an atom. Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Describe the locations, charges, and masses of the three main subatomic particles. Relative charges of −1 and +1 are assigned to the electron and proton,. Charges can. How To Find Charge Of Proton.
From sherysense.weebly.com
A proton charge sherysense How To Find Charge Of Proton Together with neutrons, they make up virtually all of the mass of an atom. Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Protons have a positive electrical charge of one (1+) and a mass. How To Find Charge Of Proton.
From mybios.me
Using The Periodic Table To Determine Protons Neutrons And Electrons How To Find Charge Of Proton Charges can be positive or negative,. To calculate the specific charge of a nucleus: Describe the locations, charges, and masses of the three main subatomic particles. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is. How To Find Charge Of Proton.
From itlessoneducation.com
What do you mean by proton charge ? It Lesson Education How To Find Charge Of Proton Charges can be positive or negative,. Describe the locations, charges, and masses of the three main subatomic particles. Together with neutrons, they make up virtually all of the mass of an atom. Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Protons have a positive electrical charge of one (1+). How To Find Charge Of Proton.
From wikihow.com
How to Find the Number of Protons, Neutrons, and Electrons How To Find Charge Of Proton Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons. Protons have a positive electrical charge of one (1+) and a mass of about 1 atomic mass unit (1 amu). Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Together with neutrons, they make up. How To Find Charge Of Proton.
From www.slideserve.com
PPT Protons are positive (+) charged particles found in the nucleus How To Find Charge Of Proton Together with neutrons, they make up virtually all of the mass of an atom. Describe the locations, charges, and masses of the three main subatomic particles. Charges can be positive or negative,. Determine the number of protons and electrons. A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and opposite to the negative charge.. How To Find Charge Of Proton.
From www.wikihow.com
How to Find the Number of Protons, Neutrons, and Electrons How To Find Charge Of Proton Specific charge (c/kg) = charge (c). Charges can be positive or negative,. Charge is measured in coulombs (c), which represent 6.242×10 18 e, where e is the charge of a proton. Specific charge is calculated by dividing the charge of a particle (in coulombs) by its mass (in kilograms): Determine the number of protons and electrons. Describe the locations, charges,. How To Find Charge Of Proton.
From www.youtube.com
How to find the number of Protons, Neutrons and Electrons? Chemistry How To Find Charge Of Proton Specific charge (c/kg) = charge (c). Charges can be positive or negative,. Determine the number of protons and electrons. Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Relative charges of −1 and +1 are assigned to the electron and proton,. Describe the locations, charges, and masses of the three main subatomic particles. Specific. How To Find Charge Of Proton.
From www.sliderbase.com
Atoms and the Periodic table Presentation Chemistry How To Find Charge Of Proton Charges can be positive or negative,. A proton is a fundamental subatomic particle with a positive electric charge equal in magnitude and opposite to the negative charge. Specific charge (c/kg) = charge (c). Relative charges of −1 and +1 are assigned to the electron and proton,. Determine the number of protons and electrons. Charge is measured in coulombs (c), which. How To Find Charge Of Proton.