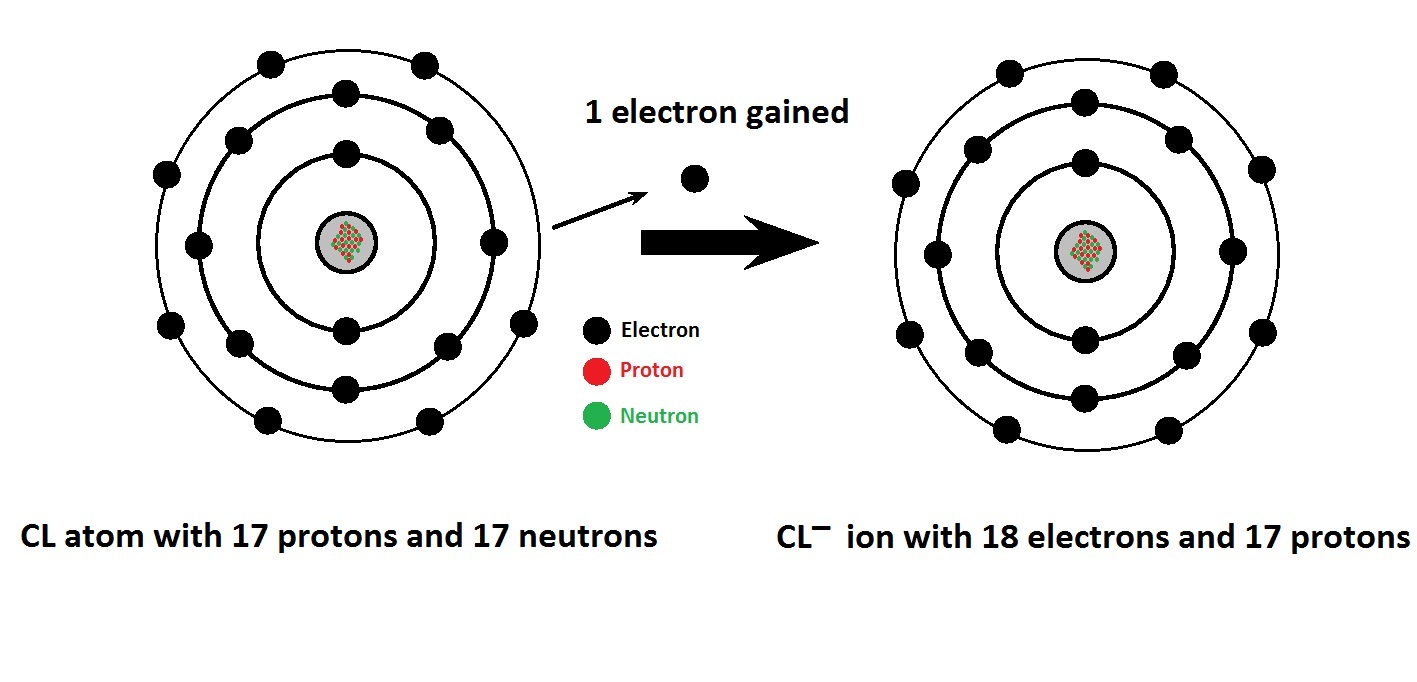
What Is The Electron Configuration Of Chlorine (Ci) . Chlorine has an atomic number of 17,. Add an electron to the anion electron configuration. Chlorine is the second lightest halogen, placed after fluorine. Electron configuration of chlorine is [ne] 3s2 3p5. For example, the ground state electronic configuration of chlorine is. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. It is an extremely reactive element and a strong oxidising agent: Among the elements, it has the. The shorthand electron configuration (or noble gas. Electron configuration chart of all elements is mentioned in the table below. It has an atomic weight of 35.450 and a mass. It has an atomic mass of 35.45 u. Full ground state electron configuration:
from basichemistry.blogspot.com
Chlorine is the second lightest halogen, placed after fluorine. It has an atomic weight of 35.450 and a mass. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). For example, the ground state electronic configuration of chlorine is. Electron configuration of chlorine is [ne] 3s2 3p5. It is an extremely reactive element and a strong oxidising agent: Chlorine has an atomic number of 17,. Full ground state electron configuration: Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Electron configuration chart of all elements is mentioned in the table below.
Basic Chemistry Ions, Cations, and Anions
What Is The Electron Configuration Of Chlorine (Ci) Full ground state electron configuration: It is an extremely reactive element and a strong oxidising agent: Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17,. Full ground state electron configuration: Among the elements, it has the. It has an atomic mass of 35.45 u. Chlorine is the second lightest halogen, placed after fluorine. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The shorthand electron configuration (or noble gas. Electron configuration chart of all elements is mentioned in the table below. For example, the ground state electronic configuration of chlorine is. Add an electron to the anion electron configuration. It has an atomic weight of 35.450 and a mass.
From sciencenotes.org
Chlorine Facts What Is The Electron Configuration Of Chlorine (Ci) Electron configuration chart of all elements is mentioned in the table below. Chlorine has an atomic number of 17,. It is an extremely reactive element and a strong oxidising agent: In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Electron configuration of chlorine is. What Is The Electron Configuration Of Chlorine (Ci).
From material-properties.org
Chlorine Protons Neutrons Electrons Electron Configuration What Is The Electron Configuration Of Chlorine (Ci) It is an extremely reactive element and a strong oxidising agent: Electron configuration of chlorine is [ne] 3s2 3p5. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. What Is The Electron Configuration Of Chlorine (Ci).
From favpng.com
Electron Configuration Aufbau Principle Valence Electron Chlorine, PNG What Is The Electron Configuration Of Chlorine (Ci) Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Electron configuration chart of all elements is mentioned in the table below. It is an extremely reactive element and a strong oxidising agent: For example, the ground state electronic configuration of chlorine is. It has an atomic weight of 35.450. What Is The Electron Configuration Of Chlorine (Ci).
From brainly.in
Draw the atomic structure of a chlorine ion Brainly.in What Is The Electron Configuration Of Chlorine (Ci) Full ground state electron configuration: In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). It has an atomic mass of 35.45 u. Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is. It is an extremely. What Is The Electron Configuration Of Chlorine (Ci).
From saylordotorg.github.io
Ions What Is The Electron Configuration Of Chlorine (Ci) Electron configuration of chlorine is [ne] 3s2 3p5. It is an extremely reactive element and a strong oxidising agent: For example, the ground state electronic configuration of chlorine is. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The shorthand electron configuration (or noble. What Is The Electron Configuration Of Chlorine (Ci).
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons What Is The Electron Configuration Of Chlorine (Ci) The shorthand electron configuration (or noble gas. Full ground state electron configuration: Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. For example, the ground state electronic configuration of chlorine is. It has an atomic weight of 35.450 and a mass. Electron configuration of chlorine is [ne] 3s2 3p5.. What Is The Electron Configuration Of Chlorine (Ci).
From egpat.com
Lewis dot structure How to write? What Is The Electron Configuration Of Chlorine (Ci) Among the elements, it has the. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The shorthand electron configuration (or noble gas. Chlorine is the second lightest halogen, placed after fluorine. Full ground state electron configuration: Add an electron to the anion electron configuration.. What Is The Electron Configuration Of Chlorine (Ci).
From www.animalia-life.club
Electron Configuration For Chlorine What Is The Electron Configuration Of Chlorine (Ci) For example, the ground state electronic configuration of chlorine is. Electron configuration of chlorine is [ne] 3s2 3p5. It is an extremely reactive element and a strong oxidising agent: It has an atomic mass of 35.45 u. Chlorine has an atomic number of 17,. The shorthand electron configuration (or noble gas. In order to write the chlorine electron configuration we. What Is The Electron Configuration Of Chlorine (Ci).
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table What Is The Electron Configuration Of Chlorine (Ci) Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. For example, the ground state electronic configuration of chlorine is. It has an atomic weight of 35.450 and a mass. It is an extremely reactive element and a strong oxidising agent: Among the elements, it has the. Full ground state. What Is The Electron Configuration Of Chlorine (Ci).
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy What Is The Electron Configuration Of Chlorine (Ci) Chlorine has an atomic number of 17,. It has an atomic mass of 35.45 u. Chlorine is the second lightest halogen, placed after fluorine. Among the elements, it has the. The shorthand electron configuration (or noble gas. For example, the ground state electronic configuration of chlorine is. It has an atomic weight of 35.450 and a mass. In order to. What Is The Electron Configuration Of Chlorine (Ci).
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica What Is The Electron Configuration Of Chlorine (Ci) For example, the ground state electronic configuration of chlorine is. Electron configuration chart of all elements is mentioned in the table below. Add an electron to the anion electron configuration. Chlorine is the second lightest halogen, placed after fluorine. Electron configuration of chlorine is [ne] 3s2 3p5. Full ground state electron configuration: In order to write the chlorine electron configuration. What Is The Electron Configuration Of Chlorine (Ci).
From animalia-life.club
Lewis Dot Structure For Chlorine What Is The Electron Configuration Of Chlorine (Ci) Chlorine has an atomic number of 17,. Electron configuration chart of all elements is mentioned in the table below. Chlorine is the second lightest halogen, placed after fluorine. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. It is an extremely reactive element and a strong oxidising agent: Among. What Is The Electron Configuration Of Chlorine (Ci).
From www.dreamstime.com
Diagram Representation Of The Element Chlorine Stock Illustration What Is The Electron Configuration Of Chlorine (Ci) For example, the ground state electronic configuration of chlorine is. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Electron configuration chart of all elements is mentioned in the table below. Add an electron to the anion electron configuration. It has an atomic mass of 35.45 u. Among the. What Is The Electron Configuration Of Chlorine (Ci).
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram What Is The Electron Configuration Of Chlorine (Ci) Chlorine has an atomic number of 17,. It is an extremely reactive element and a strong oxidising agent: Among the elements, it has the. The shorthand electron configuration (or noble gas. It has an atomic mass of 35.45 u. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Electron. What Is The Electron Configuration Of Chlorine (Ci).
From www.youtube.com
Chlorine Electron Configuration YouTube What Is The Electron Configuration Of Chlorine (Ci) Chlorine is the second lightest halogen, placed after fluorine. It has an atomic mass of 35.45 u. The shorthand electron configuration (or noble gas. It has an atomic weight of 35.450 and a mass. Among the elements, it has the. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl. What Is The Electron Configuration Of Chlorine (Ci).
From alumnos.planeaciondidactica.cucea.udg.mx
What is the electron configuration of chlorine (CI)? A) 1s22s22p3s 3p What Is The Electron Configuration Of Chlorine (Ci) In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Among the elements, it has the. Chlorine is the second lightest halogen, placed after fluorine. The shorthand electron configuration (or noble gas. For example, the ground state electronic configuration of chlorine is. Chlorine has an. What Is The Electron Configuration Of Chlorine (Ci).
From ar.inspiredpencil.com
Electron Configuration For Chlorine What Is The Electron Configuration Of Chlorine (Ci) Chlorine has an atomic number of 17,. Electron configuration of chlorine is [ne] 3s2 3p5. Full ground state electron configuration: Chlorine is the second lightest halogen, placed after fluorine. Add an electron to the anion electron configuration. It has an atomic mass of 35.45 u. For example, the ground state electronic configuration of chlorine is. It has an atomic weight. What Is The Electron Configuration Of Chlorine (Ci).
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses What Is The Electron Configuration Of Chlorine (Ci) Chlorine is the second lightest halogen, placed after fluorine. Among the elements, it has the. It has an atomic mass of 35.45 u. Full ground state electron configuration: Electron configuration of chlorine is [ne] 3s2 3p5. For example, the ground state electronic configuration of chlorine is. Chlorine is the 17th element in the periodic table and has a symbol of. What Is The Electron Configuration Of Chlorine (Ci).
From www.askiitians.com
Chlorine Study Material for IIT JEE askIITians What Is The Electron Configuration Of Chlorine (Ci) Full ground state electron configuration: For example, the ground state electronic configuration of chlorine is. The shorthand electron configuration (or noble gas. It has an atomic weight of 35.450 and a mass. Add an electron to the anion electron configuration. Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17,. Chlorine is the 17th element. What Is The Electron Configuration Of Chlorine (Ci).
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science What Is The Electron Configuration Of Chlorine (Ci) It has an atomic mass of 35.45 u. Chlorine is the second lightest halogen, placed after fluorine. The shorthand electron configuration (or noble gas. Among the elements, it has the. It is an extremely reactive element and a strong oxidising agent: Electron configuration of chlorine is [ne] 3s2 3p5. Electron configuration chart of all elements is mentioned in the table. What Is The Electron Configuration Of Chlorine (Ci).
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions What Is The Electron Configuration Of Chlorine (Ci) Chlorine is the second lightest halogen, placed after fluorine. Among the elements, it has the. Add an electron to the anion electron configuration. Electron configuration of chlorine is [ne] 3s2 3p5. It has an atomic weight of 35.450 and a mass. For example, the ground state electronic configuration of chlorine is. Chlorine is the 17th element in the periodic table. What Is The Electron Configuration Of Chlorine (Ci).
From www.chegg.com
Solved What is the electron configuration for Cl, chlorine, What Is The Electron Configuration Of Chlorine (Ci) It has an atomic weight of 35.450 and a mass. Chlorine has an atomic number of 17,. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Among the elements, it has the. It has an atomic mass of 35.45 u. Electron configuration of chlorine. What Is The Electron Configuration Of Chlorine (Ci).
From animalia-life.club
Lewis Dot Structure For Chlorine What Is The Electron Configuration Of Chlorine (Ci) It has an atomic mass of 35.45 u. Add an electron to the anion electron configuration. Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17,. Full ground state electron configuration: Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Chlorine is the second. What Is The Electron Configuration Of Chlorine (Ci).
From www.slideserve.com
PPT ELECTRONIC CONFIGURATION PowerPoint Presentation, free download What Is The Electron Configuration Of Chlorine (Ci) Electron configuration chart of all elements is mentioned in the table below. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Among the elements, it has the. Chlorine has an atomic number of 17,. It is an extremely reactive element and a strong oxidising agent: It has an atomic. What Is The Electron Configuration Of Chlorine (Ci).
From www.alamy.com
Chlorine (Cl). Diagram of the electron configuration of an atom of What Is The Electron Configuration Of Chlorine (Ci) Full ground state electron configuration: Electron configuration chart of all elements is mentioned in the table below. It has an atomic mass of 35.45 u. Electron configuration of chlorine is [ne] 3s2 3p5. Add an electron to the anion electron configuration. It is an extremely reactive element and a strong oxidising agent: In order to write the chlorine electron configuration. What Is The Electron Configuration Of Chlorine (Ci).
From stock.adobe.com
Chlorine atomic structure has atomic number, atomic mass, electron What Is The Electron Configuration Of Chlorine (Ci) For example, the ground state electronic configuration of chlorine is. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Among the elements, it has the. It has an atomic mass of 35.45 u. Chlorine is the second lightest halogen, placed after fluorine. Full ground. What Is The Electron Configuration Of Chlorine (Ci).
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille What Is The Electron Configuration Of Chlorine (Ci) Full ground state electron configuration: It has an atomic weight of 35.450 and a mass. Add an electron to the anion electron configuration. The shorthand electron configuration (or noble gas. It is an extremely reactive element and a strong oxidising agent: Chlorine is the second lightest halogen, placed after fluorine. For example, the ground state electronic configuration of chlorine is.. What Is The Electron Configuration Of Chlorine (Ci).
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram What Is The Electron Configuration Of Chlorine (Ci) Chlorine has an atomic number of 17,. Chlorine is the second lightest halogen, placed after fluorine. Electron configuration of chlorine is [ne] 3s2 3p5. Add an electron to the anion electron configuration. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Among the elements, it has the. Electron configuration. What Is The Electron Configuration Of Chlorine (Ci).
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements What Is The Electron Configuration Of Chlorine (Ci) In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Electron configuration of chlorine is [ne] 3s2 3p5. Add an electron to the anion electron. What Is The Electron Configuration Of Chlorine (Ci).
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk What Is The Electron Configuration Of Chlorine (Ci) The shorthand electron configuration (or noble gas. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Chlorine has an atomic number of 17,. Electron configuration of chlorine is [ne] 3s2 3p5. For example, the ground state electronic configuration of chlorine is. In order to write the chlorine electron configuration. What Is The Electron Configuration Of Chlorine (Ci).
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride What Is The Electron Configuration Of Chlorine (Ci) Among the elements, it has the. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. It has an atomic weight of 35.450 and a mass. Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine is the second lightest halogen, placed after fluorine. In order to write the chlorine electron. What Is The Electron Configuration Of Chlorine (Ci).
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 What Is The Electron Configuration Of Chlorine (Ci) Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. It is an extremely reactive element and a strong oxidising agent: It has an atomic mass of 35.45 u. Add an electron to the anion electron configuration. Chlorine is the second lightest halogen, placed after fluorine. Electron configuration of chlorine. What Is The Electron Configuration Of Chlorine (Ci).
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron What Is The Electron Configuration Of Chlorine (Ci) Electron configuration of chlorine is [ne] 3s2 3p5. Electron configuration chart of all elements is mentioned in the table below. Full ground state electron configuration: Add an electron to the anion electron configuration. The shorthand electron configuration (or noble gas. Among the elements, it has the. For example, the ground state electronic configuration of chlorine is. In order to write. What Is The Electron Configuration Of Chlorine (Ci).
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron What Is The Electron Configuration Of Chlorine (Ci) It is an extremely reactive element and a strong oxidising agent: The shorthand electron configuration (or noble gas. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). It has an atomic mass of 35.45 u. Full ground state electron configuration: Electron configuration of chlorine. What Is The Electron Configuration Of Chlorine (Ci).
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons What Is The Electron Configuration Of Chlorine (Ci) Full ground state electron configuration: Add an electron to the anion electron configuration. Among the elements, it has the. The shorthand electron configuration (or noble gas. Electron configuration chart of all elements is mentioned in the table below. It is an extremely reactive element and a strong oxidising agent: It has an atomic weight of 35.450 and a mass. For. What Is The Electron Configuration Of Chlorine (Ci).