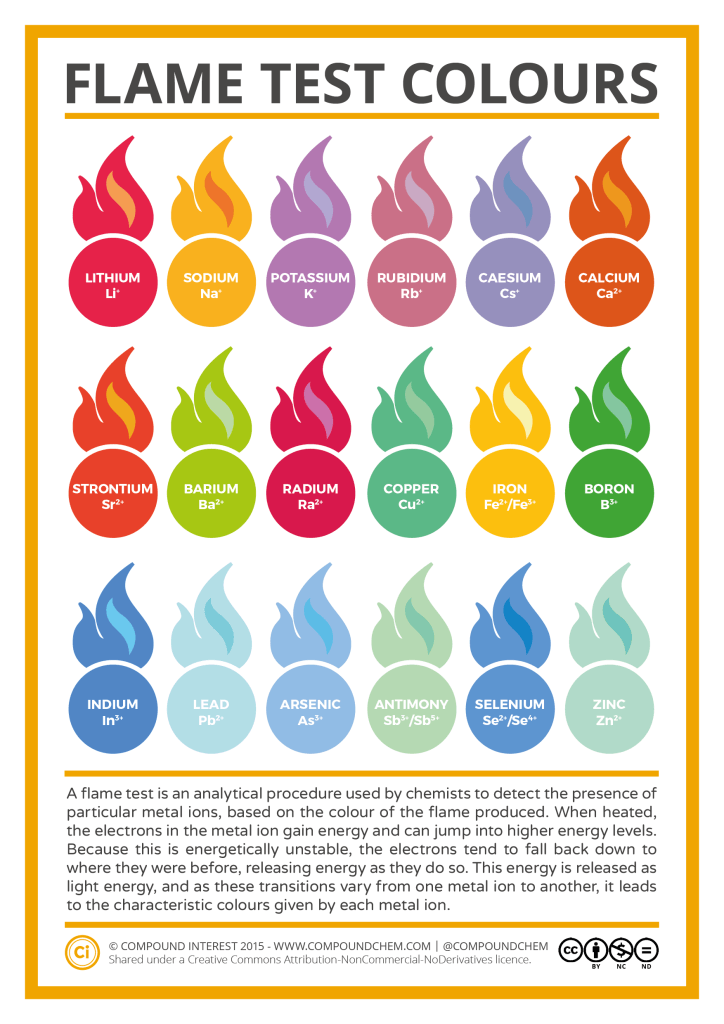
Flame Test Lab Why Different Colors . Atoms emit light when they get excited, then turn to a ground state and. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and then perform. Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. Flame tests can be used to identify some metal ions (cations). In your own words, explain how the colors. Why do you think this happens? Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. Flame tests are used to identify the presence of a. The objectives of this lab are to: Why do atoms of different elements give off different colors of light? Which pair of ions produces similar colors in the flame tests? Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and energy of the. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises.
from www.compoundchem.com
Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. Atoms emit light when they get excited, then turn to a ground state and. Flame tests can be used to identify some metal ions (cations). Which pair of ions produces similar colors in the flame tests? This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The objectives of this lab are to: Flame tests are used to identify the presence of a. In your own words, explain how the colors. Why do you think this happens?
Metal Ion Flame Test Colours Chart Compound Interest
Flame Test Lab Why Different Colors Which pair of ions produces similar colors in the flame tests? The objectives of this lab are to: Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. Flame tests are used to identify the presence of a. Flame tests can be used to identify some metal ions (cations). Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and energy of the. Why do atoms of different elements give off different colors of light? Atoms emit light when they get excited, then turn to a ground state and. Which pair of ions produces similar colors in the flame tests? This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. In your own words, explain how the colors. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and then perform. Why do you think this happens? Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame.
From ar.inspiredpencil.com
Flame Test Lab Flame Test Lab Why Different Colors The objectives of this lab are to: This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Which pair of ions produces similar colors in the flame tests? To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors. Flame Test Lab Why Different Colors.
From ctdbfredeen.blogspot.com
Lab 11 Flame Test Lab Flame Test Lab Why Different Colors Flame tests can be used to identify some metal ions (cations). Flame tests are used to identify the presence of a. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and then perform. Which pair of ions produces similar colors in the. Flame Test Lab Why Different Colors.
From myans.bhantedhammika.net
Flame Test Lab Chemistry Flame Test Lab Why Different Colors To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and then perform. Why do atoms of different elements give off different colors of light? Atoms emit light when they get excited, then turn to a ground state and. Flame tests are used. Flame Test Lab Why Different Colors.
From www.youtube.com
Flame Test Lab YouTube Flame Test Lab Why Different Colors Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. Why do atoms of different elements give off different colors of light? Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. Flame Test Lab Why Different Colors.
From www.youtube.com
Flame Test All Colors chemistry Al Chemistry English medium Flame Test Lab Why Different Colors This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Why do atoms of different elements give off different colors of light? In your own words, explain how the colors. Which pair of ions produces similar colors in the flame tests? Flame tests are used to identify. Flame Test Lab Why Different Colors.
From www.slideserve.com
PPT Chemistry in Action PowerPoint Presentation, free download ID Flame Test Lab Why Different Colors Which pair of ions produces similar colors in the flame tests? The objectives of this lab are to: To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and then perform. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame.. Flame Test Lab Why Different Colors.
From www.compoundchem.com
Metal Ion Flame Test Colours Chart Compound Interest Flame Test Lab Why Different Colors The objectives of this lab are to: To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and then perform. Atoms emit light when they get excited, then turn to a ground state and. Tables of flame test colors try to describe the. Flame Test Lab Why Different Colors.
From studylib.net
14 Flame Test lab fy11 Flame Test Lab Why Different Colors Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and energy of the. The objectives of this lab are. Flame Test Lab Why Different Colors.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Flame Test Lab Why Different Colors Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. Flame tests are used to identify the presence of a. Flame tests can be used to identify some metal ions (cations). The objectives of this lab are to: Atoms emit light when they get excited, then turn to a ground state and. Tables of flame test colors try. Flame Test Lab Why Different Colors.
From www.flipsnack.com
Flame Test Lab by Jesus Sanchez Flipsnack Flame Test Lab Why Different Colors Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and energy of the. Flame tests can be used to identify some metal ions (cations). Why do you think this happens? In your own words, explain how the colors. The objectives of this lab are to: Which pair of ions produces. Flame Test Lab Why Different Colors.
From www.slideserve.com
PPT Flame Test Lab PowerPoint Presentation, free download ID2801514 Flame Test Lab Why Different Colors Which pair of ions produces similar colors in the flame tests? Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and energy of the. Why do you think this happens? Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. Why do atoms of different elements give off. Flame Test Lab Why Different Colors.
From fireplacetips.com
Why is Fire Blue (& Is It Hotter)? Answered Fireplace Tips Flame Test Lab Why Different Colors In your own words, explain how the colors. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. The objectives of this lab are to: Why do atoms of different elements give off different colors of light? Flame tests can be used to identify some metal ions (cations). Atoms emit light when they get excited, then turn to. Flame Test Lab Why Different Colors.
From www.youtube.com
Flame Test Lab YouTube Flame Test Lab Why Different Colors Which pair of ions produces similar colors in the flame tests? Flame tests are used to identify the presence of a. Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. To perform flame tests of metal cations in. Flame Test Lab Why Different Colors.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID3925939 Flame Test Lab Why Different Colors Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. Flame tests can be used to identify some metal ions (cations). Flame tests are used to identify the presence of a. Why do atoms of different elements give off. Flame Test Lab Why Different Colors.
From www.dreamstime.com
Flame Test for Different Metal Produces Different Color Flame Stock Flame Test Lab Why Different Colors Atoms emit light when they get excited, then turn to a ground state and. Why do atoms of different elements give off different colors of light? Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. Flame tests are used to identify the presence of a. Which pair of ions produces similar colors in the flame tests? This. Flame Test Lab Why Different Colors.
From hightechhigh-faithsdp.weebly.com
Flame Test Lab Faith's DP Flame Test Lab Why Different Colors Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and energy of the. Flame tests are used to identify the presence of a. Flame tests can be used to identify some metal ions (cations). Tables of flame test colors try to describe the hue of each flame as accurately as. Flame Test Lab Why Different Colors.
From materialfullpartible.z21.web.core.windows.net
Lab Report Flame Test Flame Test Lab Why Different Colors In your own words, explain how the colors. Why do you think this happens? Which pair of ions produces similar colors in the flame tests? To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and then perform. Why do atoms of different. Flame Test Lab Why Different Colors.
From www.vecteezy.com
Flame test of different metal produces different color flame 20240736 Flame Test Lab Why Different Colors Why do you think this happens? Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. In your own words, explain how the colors. Flame tests can be used to identify some metal ions (cations). Which pair of ions. Flame Test Lab Why Different Colors.
From www.bbc.com
Chemical analysis Revision 2 National 5 Chemistry BBC Bitesize Flame Test Lab Why Different Colors Why do atoms of different elements give off different colors of light? Atoms emit light when they get excited, then turn to a ground state and. Flame tests can be used to identify some metal ions (cations). Flame tests are used to identify the presence of a. In your own words, explain how the colors. Which pair of ions produces. Flame Test Lab Why Different Colors.
From alexbenavidezchem.wordpress.com
Flame Test Lab! alexbenavidezchem Flame Test Lab Why Different Colors Why do atoms of different elements give off different colors of light? Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. Flame tests are used to identify the presence of a. In your own words, explain how the. Flame Test Lab Why Different Colors.
From mucholderthen.tumblr.com
Science Visualized • Flame color of various elements as compounds in... Flame Test Lab Why Different Colors Why do atoms of different elements give off different colors of light? The objectives of this lab are to: Atoms emit light when they get excited, then turn to a ground state and. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light,. Flame Test Lab Why Different Colors.
From ar.inspiredpencil.com
Flame Test Sodium Flame Test Lab Why Different Colors Flame tests can be used to identify some metal ions (cations). In your own words, explain how the colors. Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours. Flame Test Lab Why Different Colors.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Flame Test Lab Why Different Colors Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. Flame tests are used to identify the presence of a. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. Which pair of ions produces similar colors. Flame Test Lab Why Different Colors.
From www.vectorstock.com
Flame test of different metal produces different Vector Image Flame Test Lab Why Different Colors Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. Why do atoms of different elements give off different colors of light? Flame tests are used to identify the presence of a. In your own words, explain how the. Flame Test Lab Why Different Colors.
From www.youtube.com
Experiment 5 colored flames (How to color fire with salts) YouTube Flame Test Lab Why Different Colors To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and then perform. The objectives of this lab are to: Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and energy of the.. Flame Test Lab Why Different Colors.
From sciencelessonsthatrock.com
Chemistry Flame Test Lab Science Lessons That Rock Flame Test Lab Why Different Colors Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and energy of the. Flame tests are used to identify the presence of a. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. To perform flame tests. Flame Test Lab Why Different Colors.
From www.youtube.com
Chemistry Flame Test Lab Flame Test Experiment Flame Test Colours Flame Test Lab Why Different Colors Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and energy of the. Atoms emit light when they get excited, then turn to a ground state and. Flame tests can be used to identify some metal ions (cations). Tables of flame test colors try to describe the hue of each. Flame Test Lab Why Different Colors.
From sciencelessonsthatrock.com
Chemistry Flame Test Lab Science Lessons That Rock Flame Test Lab Why Different Colors In your own words, explain how the colors. Why do you think this happens? Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. Atoms emit light when they get excited, then turn to a ground state and. Why do atoms of different elements give off different colors of light? Which pair of ions produces similar colors in. Flame Test Lab Why Different Colors.
From www.alamy.com
Iron chloride Stock Vector Images Alamy Flame Test Lab Why Different Colors The objectives of this lab are to: Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of crayola crayons. Atoms emit light when they get excited, then turn to a ground state and. Why do atoms of different elements give off. Flame Test Lab Why Different Colors.
From www.animalia-life.club
Flame Test Lab And Fireworks Clipart Flame Test Lab Why Different Colors Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and energy of the. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and then perform. In your own words, explain how the. Flame Test Lab Why Different Colors.
From kelseyreavy.com
Why You Need to do the Flame Test Lab in Your High School Chemistry Flame Test Lab Why Different Colors To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and then perform. Atoms emit light when they get excited, then turn to a ground state and. Which pair of ions produces similar colors in the flame tests? Why do atoms of different. Flame Test Lab Why Different Colors.
From slideplayer.com
Light emissions minilab Pt 1 Flame test Wrap up ppt download Flame Test Lab Why Different Colors To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and then perform. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and energy of the. The objectives of this lab are to:. Flame Test Lab Why Different Colors.
From slideplayer.com
Flame Test Lab. ppt download Flame Test Lab Why Different Colors In your own words, explain how the colors. Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. Which pair of ions produces similar colors in the flame tests? Why do you think this happens? The objectives of this lab are to: To perform flame tests of metal cations in order to observe their characteristic colors, to match. Flame Test Lab Why Different Colors.
From slideplayer.com
WarmUp 11/18/14 Draw the Lewis dot structure of a phosphorus atom Flame Test Lab Why Different Colors Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and energy of the. Why do you think this happens? Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors. Flame Test Lab Why Different Colors.
From in.pinterest.com
Learn how to make colored fire at home in your fireplace or campfire Flame Test Lab Why Different Colors In your own words, explain how the colors. This page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Tables of flame test colors try to describe the hue of each flame as accurately as possible, so you'll see color names rivaling those of the big box of. Flame Test Lab Why Different Colors.