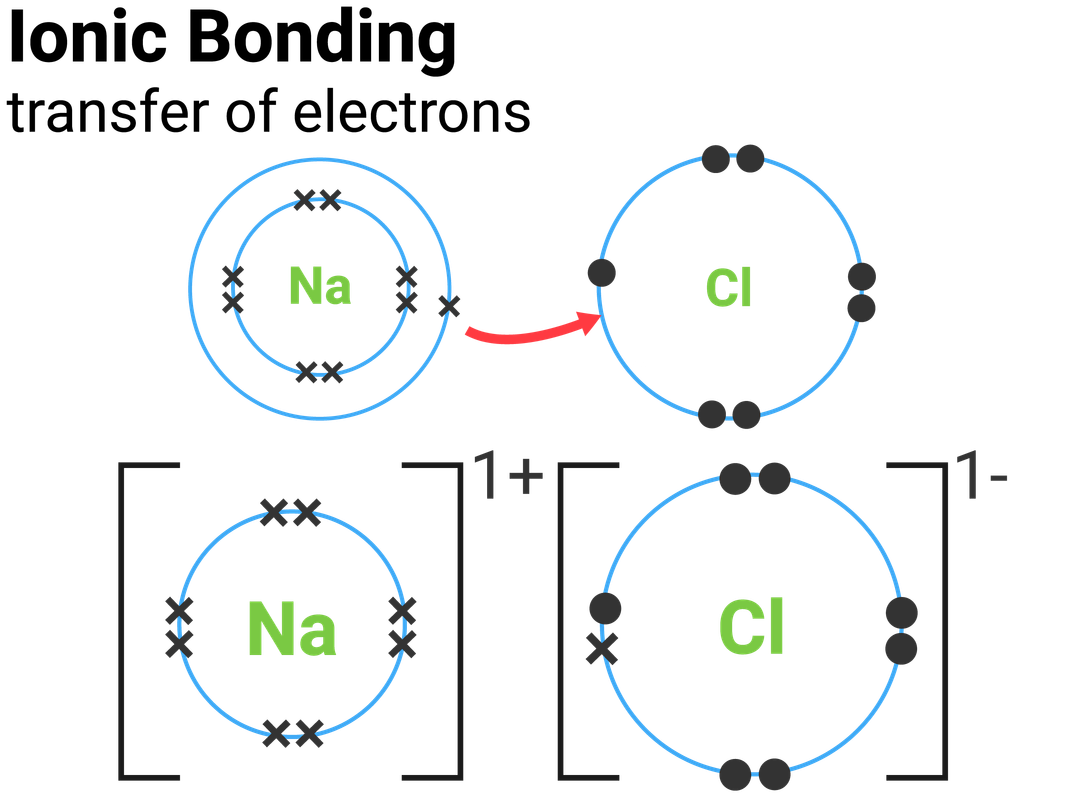
Is Salt Ionic Or Covalent . Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Learn the difference between ionic and covalent bonds, how they form, and their properties. Polar covalent is the intermediate type of bonding between the two extremes. Learn the difference between ionic and covalent bonds, and see examples of each. Many bonds can be covalent in one situation and ionic in another. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Salt is an example of an ionic bond between a metal and a nonmetal. The classic example is table salt or sodium chloride. Sodium chloride is an ionic compound. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Ionic and covalent bonds are the two extremes of bonding.
from revisechemistry.uk
Many bonds can be covalent in one situation and ionic in another. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Salt is an example of an ionic bond between a metal and a nonmetal. Ionic and covalent bonds are the two extremes of bonding. Learn the difference between ionic and covalent bonds, and see examples of each. Polar covalent is the intermediate type of bonding between the two extremes. The classic example is table salt or sodium chloride. Sodium chloride is an ionic compound. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and.
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
Is Salt Ionic Or Covalent The classic example is table salt or sodium chloride. The classic example is table salt or sodium chloride. Learn the difference between ionic and covalent bonds, how they form, and their properties. Many bonds can be covalent in one situation and ionic in another. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Sodium chloride is an ionic compound. Polar covalent is the intermediate type of bonding between the two extremes. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Salt is an example of an ionic bond between a metal and a nonmetal. Ionic and covalent bonds are the two extremes of bonding. Learn the difference between ionic and covalent bonds, and see examples of each.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Is Salt Ionic Or Covalent Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Salt is an example of an ionic bond between a metal and a nonmetal. Learn the difference between ionic and covalent bonds, and see examples of each. Learn the difference between ionic. Is Salt Ionic Or Covalent.
From www.youtube.com
Ionic and Covalent Bonding Chemistry YouTube Is Salt Ionic Or Covalent Many bonds can be covalent in one situation and ionic in another. Sodium chloride is an ionic compound. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Learn the difference between ionic and covalent bonds, and see examples of each. Learn the difference between ionic and covalent bonds, how they form, and their properties. In chemistry,. Is Salt Ionic Or Covalent.
From www.youtube.com
Is NaCl(Sodium chloride or Table salt) ionic or covalent? YouTube Is Salt Ionic Or Covalent For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Many bonds can be covalent in one situation and ionic in another. Salt is an example of an ionic. Is Salt Ionic Or Covalent.
From www.slideserve.com
PPT 2.3 Classifying Chemical Compounds Properties of Ionic and Is Salt Ionic Or Covalent Sodium chloride is an ionic compound. Salt is an example of an ionic bond between a metal and a nonmetal. Learn the difference between ionic and covalent bonds, how they form, and their properties. Many bonds can be covalent in one situation and ionic in another. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Ionic. Is Salt Ionic Or Covalent.
From techiescientist.com
Is KCl Covalent or Ionic? Techiescientist Is Salt Ionic Or Covalent Learn the difference between ionic and covalent bonds, and see examples of each. Learn the difference between ionic and covalent bonds, how they form, and their properties. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. For instance, hydrogen chloride, hcl,. Is Salt Ionic Or Covalent.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Is Salt Ionic Or Covalent Sodium chloride is an ionic compound. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Salt is an example of an ionic bond between a metal and a nonmetal. Many bonds can be covalent in one situation and ionic in another. Ionic and covalent bonds are the two extremes of bonding. The classic example is table. Is Salt Ionic Or Covalent.
From sciencenotes.org
Examples of Ionic Compounds in Everyday Life Is Salt Ionic Or Covalent For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Learn the difference between ionic and covalent bonds, how they form, and their properties. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Many bonds can be covalent. Is Salt Ionic Or Covalent.
From www.chemistrystudent.com
Covalent and Ionic Character (ALevel) ChemistryStudent Is Salt Ionic Or Covalent In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Learn the difference between ionic and covalent bonds, and see examples of each. Polar covalent is the intermediate type of bonding between the two extremes. Many bonds can be covalent in one situation and ionic in another. The classic example. Is Salt Ionic Or Covalent.
From techiescientist.com
Is SO3 Ionic or Covalent? Techiescientist Is Salt Ionic Or Covalent Learn the difference between ionic and covalent bonds, and see examples of each. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Ionic and covalent bonds are the two extremes of bonding. Many bonds can be covalent in one situation and. Is Salt Ionic Or Covalent.
From ar.inspiredpencil.com
Covalent Bonding Vs Ionic Bonding Is Salt Ionic Or Covalent Polar covalent is the intermediate type of bonding between the two extremes. Salt is an example of an ionic bond between a metal and a nonmetal. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Ionic. Is Salt Ionic Or Covalent.
From slideplayer.com
Chemical Bonding Ionic and Covalent. ppt download Is Salt Ionic Or Covalent Salt is an example of an ionic bond between a metal and a nonmetal. The classic example is table salt or sodium chloride. Learn the difference between ionic and covalent bonds, and see examples of each. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Sodium chloride is an ionic compound. Covalent bonds are the attractive. Is Salt Ionic Or Covalent.
From maritzaqogallagher.blogspot.com
Is Nacl Ionic or Covalent MaritzaqoGallagher Is Salt Ionic Or Covalent Learn the difference between ionic and covalent bonds, how they form, and their properties. Salt is an example of an ionic bond between a metal and a nonmetal. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Ionic and covalent bonds. Is Salt Ionic Or Covalent.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID6901289 Is Salt Ionic Or Covalent In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Ionic and covalent bonds are the two extremes of bonding. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one. Is Salt Ionic Or Covalent.
From fourthgradegc.blogspot.com
Fourth Grade GC August 2013 Is Salt Ionic Or Covalent Learn the difference between ionic and covalent bonds, and see examples of each. Salt is an example of an ionic bond between a metal and a nonmetal. Ionic and covalent bonds are the two extremes of bonding. The classic example is table salt or sodium chloride. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded. Is Salt Ionic Or Covalent.
From techiescientist.com
Is SiO2 Ionic or Covalent? Techiescientist Is Salt Ionic Or Covalent Sodium chloride is an ionic compound. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Many bonds can be covalent in one situation and ionic in another. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and. Is Salt Ionic Or Covalent.
From examples.yourdictionary.com
Main Differences Between Ionic and Covalent Bonds YourDictionary Is Salt Ionic Or Covalent Many bonds can be covalent in one situation and ionic in another. Ionic and covalent bonds are the two extremes of bonding. Learn the difference between ionic and covalent bonds, and see examples of each. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located. Is Salt Ionic Or Covalent.
From chem.libretexts.org
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts Is Salt Ionic Or Covalent Salt is an example of an ionic bond between a metal and a nonmetal. Many bonds can be covalent in one situation and ionic in another. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Polar covalent is the intermediate type of bonding between the two extremes. Learn the difference between ionic and covalent bonds, how. Is Salt Ionic Or Covalent.
From pediaa.com
Difference Between Covalent and Ionic Bonds Is Salt Ionic Or Covalent Learn the difference between ionic and covalent bonds, how they form, and their properties. Polar covalent is the intermediate type of bonding between the two extremes. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Sodium. Is Salt Ionic Or Covalent.
From stock.adobe.com
Ionic vs. Covalent Compounds in an aqueous solution. Sugar and Salt Is Salt Ionic Or Covalent Salt is an example of an ionic bond between a metal and a nonmetal. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Ionic and covalent bonds are the two extremes of bonding. Learn the difference between ionic and covalent bonds,. Is Salt Ionic Or Covalent.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities Is Salt Ionic Or Covalent The classic example is table salt or sodium chloride. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Polar covalent is the intermediate type of bonding between the two extremes. Sodium chloride is an ionic compound. Covalent bonds are the attractive forces between the positively charged nuclei of the. Is Salt Ionic Or Covalent.
From sciencenotes.org
Ionic vs Covalent Bonds Is Salt Ionic Or Covalent Sodium chloride is an ionic compound. Learn the difference between ionic and covalent bonds, and see examples of each. Ionic and covalent bonds are the two extremes of bonding. Polar covalent is the intermediate type of bonding between the two extremes. Many bonds can be covalent in one situation and ionic in another. The classic example is table salt or. Is Salt Ionic Or Covalent.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Is Salt Ionic Or Covalent The classic example is table salt or sodium chloride. Learn the difference between ionic and covalent bonds, and see examples of each. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Sodium chloride is an ionic compound. Learn the difference between ionic and covalent bonds, how they form, and their properties. Many bonds can be covalent. Is Salt Ionic Or Covalent.
From slideplayer.com
Covalent Bonding. ppt download Is Salt Ionic Or Covalent Many bonds can be covalent in one situation and ionic in another. The classic example is table salt or sodium chloride. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Learn the difference between ionic and covalent bonds, and see examples of each. Ionic and covalent bonds are the two extremes of bonding. Sodium chloride is. Is Salt Ionic Or Covalent.
From en.wikipedia.org
Ionic bonding Wikipedia Is Salt Ionic Or Covalent In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Learn the difference between ionic and covalent bonds, how they form, and their properties. Many bonds can be covalent in one situation and ionic in another. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Covalent. Is Salt Ionic Or Covalent.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Is Salt Ionic Or Covalent Learn the difference between ionic and covalent bonds, and see examples of each. Polar covalent is the intermediate type of bonding between the two extremes. Many bonds can be covalent in one situation and ionic in another. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Learn the difference between ionic and covalent bonds, how they. Is Salt Ionic Or Covalent.
From topblogtenz.com
Is MgCl2 ionic or covalent? Nature of chemical bond in MgCl2 Is Salt Ionic Or Covalent Polar covalent is the intermediate type of bonding between the two extremes. Many bonds can be covalent in one situation and ionic in another. Ionic and covalent bonds are the two extremes of bonding. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between. Is Salt Ionic Or Covalent.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Is Salt Ionic Or Covalent In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. Learn the difference between ionic and covalent bonds, and see examples of each. The classic example is table salt or sodium chloride. Salt is an example of an ionic bond between a metal and a nonmetal. Polar covalent is the. Is Salt Ionic Or Covalent.
From brainly.com
This image shows the ionic bond between sodium and chloride in NaCl Is Salt Ionic Or Covalent Salt is an example of an ionic bond between a metal and a nonmetal. In chemistry, a salt is an electrically neutral chemical compound consisting of cations and anions connected by an ionic bond. The classic example is table salt or sodium chloride. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Learn the difference between. Is Salt Ionic Or Covalent.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii Is Salt Ionic Or Covalent For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Sodium chloride is an ionic compound. Many bonds can be covalent in one situation and ionic in another. Learn the difference between ionic and covalent bonds, and see examples of each. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and. Is Salt Ionic Or Covalent.
From slideplayer.com
Crystal Binding (Bonding) Part III ppt download Is Salt Ionic Or Covalent Many bonds can be covalent in one situation and ionic in another. Learn the difference between ionic and covalent bonds, and see examples of each. Salt is an example of an ionic bond between a metal and a nonmetal. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Is Salt Ionic Or Covalent.
From thisonevsthatone.com
Ionic vs Covalent Which is which and how to tell them apart Is Salt Ionic Or Covalent Learn the difference between ionic and covalent bonds, and see examples of each. Learn the difference between ionic and covalent bonds, how they form, and their properties. Polar covalent is the intermediate type of bonding between the two extremes. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and. Many bonds can be covalent in one situation. Is Salt Ionic Or Covalent.
From techiescientist.com
Is NH4Cl Ionic or Covalent? Techiescientist Is Salt Ionic Or Covalent Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Ionic and covalent bonds are the two extremes of bonding. Sodium chloride is an ionic compound. Learn the difference between ionic and covalent bonds, how they form, and their properties. For instance,. Is Salt Ionic Or Covalent.
From www.dreamstime.com
Ionic Stock Illustrations 4,680 Ionic Stock Illustrations, Vectors Is Salt Ionic Or Covalent Learn the difference between ionic and covalent bonds, how they form, and their properties. Many bonds can be covalent in one situation and ionic in another. Polar covalent is the intermediate type of bonding between the two extremes. The classic example is table salt or sodium chloride. For instance, hydrogen chloride, hcl, is a gas in which the hydrogen and.. Is Salt Ionic Or Covalent.
From www.youtube.com
How to tell if Ionic Bond or Covalent Bond YouTube Is Salt Ionic Or Covalent Learn the difference between ionic and covalent bonds, how they form, and their properties. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Polar covalent is the intermediate type of bonding between the two extremes. Ionic and covalent bonds are the. Is Salt Ionic Or Covalent.
From www.youtube.com
Is Table Salt (NaCl ) Ionic or Covalent/Molecular? YouTube Is Salt Ionic Or Covalent The classic example is table salt or sodium chloride. Many bonds can be covalent in one situation and ionic in another. Polar covalent is the intermediate type of bonding between the two extremes. Sodium chloride is an ionic compound. Ionic and covalent bonds are the two extremes of bonding. Salt is an example of an ionic bond between a metal. Is Salt Ionic Or Covalent.