How To Calculate The Osmolality Of A Solution . To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). An osmole is 1 mol of particles that contribute to the osmotic. Steps required to measure the osmolarity of a solution: An osmole (osmol) is 1 mol of particles that. Now calculate the molarity of the solution. Osmolarity is the number of osmoles of solute per litre of solution. Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): Convert that number to osmoles per liter and add the osmolarity of each. You multiply the molarity by the number of osmoles that each solute produces. Calculate the moles of a solute dissolved in a solution.
from www.chegg.com
Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). Steps required to measure the osmolarity of a solution: An osmole (osmol) is 1 mol of particles that. Convert that number to osmoles per liter and add the osmolarity of each. Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): Osmolarity is the number of osmoles of solute per litre of solution. Calculate the moles of a solute dissolved in a solution. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. An osmole is 1 mol of particles that contribute to the osmotic. You multiply the molarity by the number of osmoles that each solute produces.
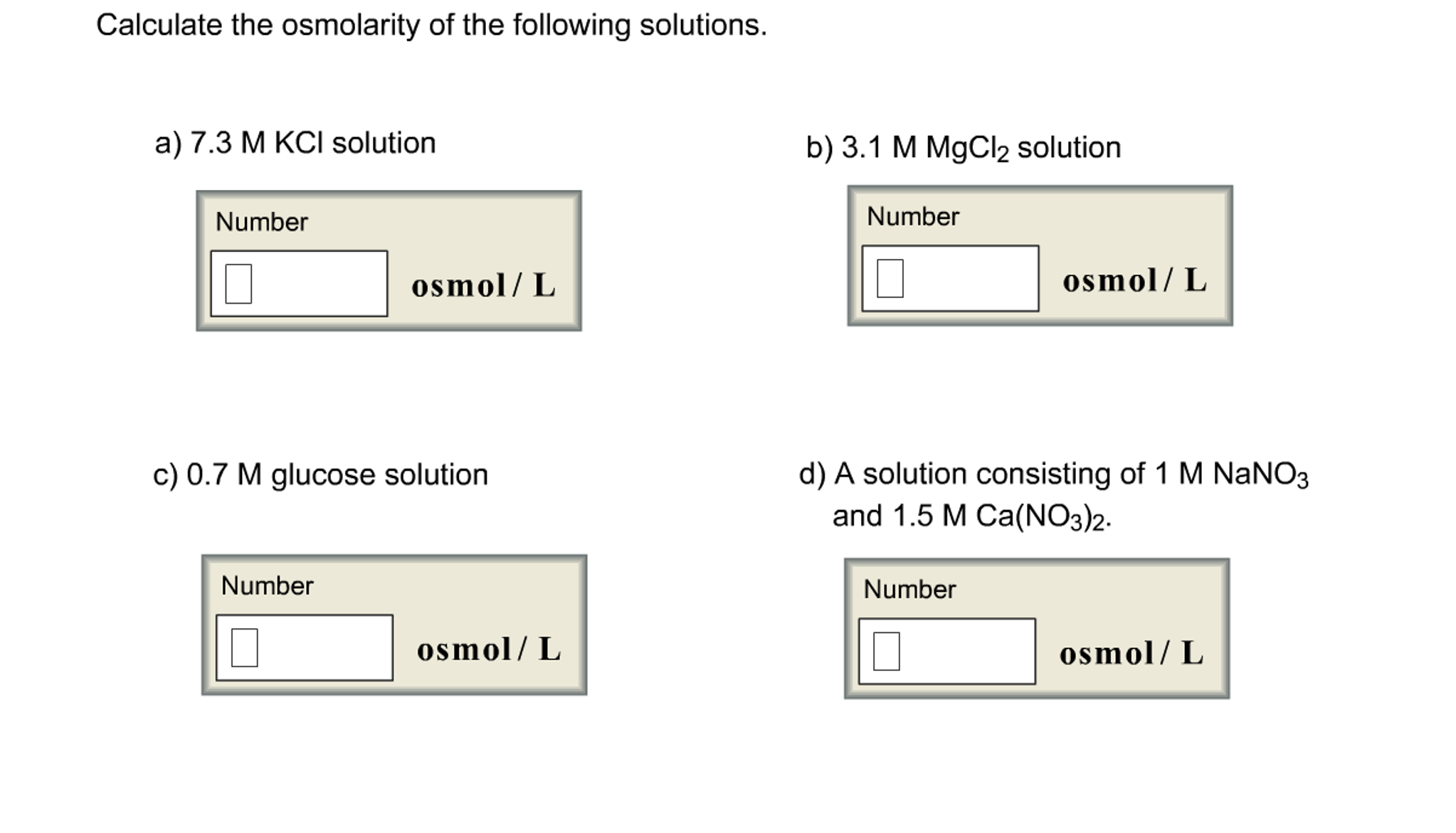
Solved Calculate the osmolarity of the following solutions.
How To Calculate The Osmolality Of A Solution Calculate the moles of a solute dissolved in a solution. Osmolarity is the number of osmoles of solute per litre of solution. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). Now calculate the molarity of the solution. Steps required to measure the osmolarity of a solution: Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): An osmole is 1 mol of particles that contribute to the osmotic. Calculate the moles of a solute dissolved in a solution. An osmole (osmol) is 1 mol of particles that. Convert that number to osmoles per liter and add the osmolarity of each. You multiply the molarity by the number of osmoles that each solute produces. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter.
From www.youtube.com
Osmolarity Example (Bio) YouTube How To Calculate The Osmolality Of A Solution Osmolarity is the number of osmoles of solute per litre of solution. An osmole (osmol) is 1 mol of particles that. You multiply the molarity by the number of osmoles that each solute produces. Now calculate the molarity of the solution. Convert that number to osmoles per liter and add the osmolarity of each. To calculate the osmolarity of a. How To Calculate The Osmolality Of A Solution.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download How To Calculate The Osmolality Of A Solution To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Calculate the moles of a solute dissolved in a solution. You multiply the molarity by the number of osmoles that each solute produces. Osmolarity is the number of osmoles of solute per litre of solution. An osmole (osmol) is 1 mol. How To Calculate The Osmolality Of A Solution.
From www.numerade.com
SOLVED Calculate the Osmolarity and Osmolality of NaCl in 0.9 (w/v How To Calculate The Osmolality Of A Solution Now calculate the molarity of the solution. Steps required to measure the osmolarity of a solution: Calculate the moles of a solute dissolved in a solution. An osmole (osmol) is 1 mol of particles that. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). Convert that number to osmoles. How To Calculate The Osmolality Of A Solution.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the How To Calculate The Osmolality Of A Solution Convert that number to osmoles per liter and add the osmolarity of each. Now calculate the molarity of the solution. Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): Osmolarity is the number of osmoles of solute per litre of solution. You multiply the molarity by the number. How To Calculate The Osmolality Of A Solution.
From www.coursehero.com
[Solved] I need help figuring out how to calculate molality and How To Calculate The Osmolality Of A Solution An osmole (osmol) is 1 mol of particles that. Now calculate the molarity of the solution. Calculate the moles of a solute dissolved in a solution. Osmolarity is the number of osmoles of solute per litre of solution. An osmole is 1 mol of particles that contribute to the osmotic. Convert that number to osmoles per liter and add the. How To Calculate The Osmolality Of A Solution.
From www.youtube.com
Calculate your own osmolarity Lab values and concentrations Health How To Calculate The Osmolality Of A Solution You multiply the molarity by the number of osmoles that each solute produces. Calculate the moles of a solute dissolved in a solution. Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): Osmolarity is the number of osmoles of solute per litre of solution. Convert that number to. How To Calculate The Osmolality Of A Solution.
From www.chegg.com
Solved Problems Calculate the osmolality of the following How To Calculate The Osmolality Of A Solution You multiply the molarity by the number of osmoles that each solute produces. An osmole is 1 mol of particles that contribute to the osmotic. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). To calculate the osmolarity of a solution, the first step is to convert the number. How To Calculate The Osmolality Of A Solution.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube How To Calculate The Osmolality Of A Solution Steps required to measure the osmolarity of a solution: Osmolarity is the number of osmoles of solute per litre of solution. Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): To calculate the osmolarity of a solution, the first step is to convert the number to moles per. How To Calculate The Osmolality Of A Solution.
From www.slideserve.com
PPT Water Metabolism PowerPoint Presentation, free download ID4493121 How To Calculate The Osmolality Of A Solution Now calculate the molarity of the solution. Calculate the moles of a solute dissolved in a solution. Osmolarity is the number of osmoles of solute per litre of solution. An osmole is 1 mol of particles that contribute to the osmotic. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity. How To Calculate The Osmolality Of A Solution.
From www.youtube.com
How to solve osmolarity calculation problems 3 YouTube How To Calculate The Osmolality Of A Solution Calculate the moles of a solute dissolved in a solution. Steps required to measure the osmolarity of a solution: Convert that number to osmoles per liter and add the osmolarity of each. Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): Now calculate the molarity of the solution.. How To Calculate The Osmolality Of A Solution.
From ojklmexhbr.blogspot.com
How To Calculate Serum Osmolality In this study, we compared the How To Calculate The Osmolality Of A Solution You multiply the molarity by the number of osmoles that each solute produces. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Now calculate the molarity of the solution. Calculate the moles of a solute dissolved in a solution. Steps required to measure the osmolarity of a solution: Unless a. How To Calculate The Osmolality Of A Solution.
From www.thetechedvocate.org
How to calculate osmolality The Tech Edvocate How To Calculate The Osmolality Of A Solution To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Calculate the moles of a solute dissolved in a solution. Osmolarity is the number of osmoles of solute per litre of solution. Now calculate the molarity of the solution. Steps required to measure the osmolarity of a solution: Unless a solution. How To Calculate The Osmolality Of A Solution.
From www.researchgate.net
The osmolality of solutions. Download Table How To Calculate The Osmolality Of A Solution Calculate the moles of a solute dissolved in a solution. An osmole is 1 mol of particles that contribute to the osmotic. Osmolarity is the number of osmoles of solute per litre of solution. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). Convert that number to osmoles per. How To Calculate The Osmolality Of A Solution.
From pediaa.com
Difference Between Osmolarity and Osmolality Definition, Explanation How To Calculate The Osmolality Of A Solution Osmolarity is the number of osmoles of solute per litre of solution. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): An osmole (osmol) is 1 mol of particles. How To Calculate The Osmolality Of A Solution.
From www.reddit.com
How to calculate the osmolality of 0.1 M solution of Na2HPO4 + NaH2PO4 How To Calculate The Osmolality Of A Solution You multiply the molarity by the number of osmoles that each solute produces. Calculate the moles of a solute dissolved in a solution. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Steps required to measure the osmolarity of a solution: Multiply the number of particles produced from dissolving the. How To Calculate The Osmolality Of A Solution.
From www.youtube.com
Calculating the Osmolarity of a Solution (Question 2) YouTube How To Calculate The Osmolality Of A Solution To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): Calculate the moles of a solute dissolved in a solution. Osmolarity is the number of osmoles of solute per litre. How To Calculate The Osmolality Of A Solution.
From www.youtube.com
How to solve osmolarity calculation problems 4 YouTube How To Calculate The Osmolality Of A Solution Convert that number to osmoles per liter and add the osmolarity of each. Osmolarity is the number of osmoles of solute per litre of solution. Steps required to measure the osmolarity of a solution: You multiply the molarity by the number of osmoles that each solute produces. An osmole (osmol) is 1 mol of particles that. To calculate the osmolarity. How To Calculate The Osmolality Of A Solution.
From www.youtube.com
Calculating the Osmolarity of a Solution YouTube How To Calculate The Osmolality Of A Solution An osmole (osmol) is 1 mol of particles that. Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): Calculate the moles of a solute dissolved in a solution. Now calculate the molarity of the solution. An osmole is 1 mol of particles that contribute to the osmotic. To. How To Calculate The Osmolality Of A Solution.
From droualb.faculty.mjc.edu
Chapter 4 How To Calculate The Osmolality Of A Solution Now calculate the molarity of the solution. Steps required to measure the osmolarity of a solution: To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. An osmole is 1 mol of particles that contribute to the osmotic. Unless a solution is very concentrated, the osmolarity of a solution (c) can. How To Calculate The Osmolality Of A Solution.
From www.youtube.com
How to solve osmolarity calculation problems 2 YouTube How To Calculate The Osmolality Of A Solution You multiply the molarity by the number of osmoles that each solute produces. An osmole is 1 mol of particles that contribute to the osmotic. Calculate the moles of a solute dissolved in a solution. Osmolarity is the number of osmoles of solute per litre of solution. Now calculate the molarity of the solution. Multiply the number of particles produced. How To Calculate The Osmolality Of A Solution.
From www.coursehero.com
[Solved] I need help figuring out how to calculate molality and How To Calculate The Osmolality Of A Solution An osmole is 1 mol of particles that contribute to the osmotic. Steps required to measure the osmolarity of a solution: An osmole (osmol) is 1 mol of particles that. Now calculate the molarity of the solution. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Unless a solution is. How To Calculate The Osmolality Of A Solution.
From www.chegg.com
Solved Concentration Osmolarity . Ca 2.12.8 How To Calculate The Osmolality Of A Solution Calculate the moles of a solute dissolved in a solution. Osmolarity is the number of osmoles of solute per litre of solution. An osmole (osmol) is 1 mol of particles that. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). Steps required to measure the osmolarity of a solution:. How To Calculate The Osmolality Of A Solution.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 1. Osmosis ditki medical How To Calculate The Osmolality Of A Solution Now calculate the molarity of the solution. Osmolarity is the number of osmoles of solute per litre of solution. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). Calculate the moles of a solute dissolved in a solution. Convert that number to osmoles per liter and add the osmolarity. How To Calculate The Osmolality Of A Solution.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 2. Osmolarity ditki How To Calculate The Osmolality Of A Solution Now calculate the molarity of the solution. Osmolarity is the number of osmoles of solute per litre of solution. Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol).. How To Calculate The Osmolality Of A Solution.
From www.youtube.com
Calculated Osmolality YouTube How To Calculate The Osmolality Of A Solution An osmole is 1 mol of particles that contribute to the osmotic. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). Convert that number to osmoles per liter and add the osmolarity of each. Now calculate the molarity of the solution. Osmolarity is the number of osmoles of solute. How To Calculate The Osmolality Of A Solution.
From ibiologia.com
How to calculate Osmolarity from Molarity? How To Calculate The Osmolality Of A Solution Steps required to measure the osmolarity of a solution: An osmole (osmol) is 1 mol of particles that. Calculate the moles of a solute dissolved in a solution. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). Osmolarity is the number of osmoles of solute per litre of solution.. How To Calculate The Osmolality Of A Solution.
From people.tamu.edu
http//dl.clackamas.edu/ch10504/calculat.htm How To Calculate The Osmolality Of A Solution Osmolarity is the number of osmoles of solute per litre of solution. An osmole is 1 mol of particles that contribute to the osmotic. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Now calculate the molarity of the solution. Multiply the number of particles produced from dissolving the solution. How To Calculate The Osmolality Of A Solution.
From www.chegg.com
Solved Calculate the osmolarity of the following solutions. How To Calculate The Osmolality Of A Solution Now calculate the molarity of the solution. Osmolarity is the number of osmoles of solute per litre of solution. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). You multiply the molarity by the number of osmoles that each solute produces. An osmole is 1 mol of particles that. How To Calculate The Osmolality Of A Solution.
From www.chegg.com
Solved Question 3 Calculate the osmolarity of the following How To Calculate The Osmolality Of A Solution An osmole is 1 mol of particles that contribute to the osmotic. Convert that number to osmoles per liter and add the osmolarity of each. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated. How To Calculate The Osmolality Of A Solution.
From www.youtube.com
Estimating Serum Osmolality using a Simple Formula YouTube How To Calculate The Osmolality Of A Solution You multiply the molarity by the number of osmoles that each solute produces. An osmole is 1 mol of particles that contribute to the osmotic. An osmole (osmol) is 1 mol of particles that. Now calculate the molarity of the solution. To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter.. How To Calculate The Osmolality Of A Solution.
From www.reddit.com
How to calculate the osmolality of 0.1 M solution of Na2HPO4 + NaH2PO4 How To Calculate The Osmolality Of A Solution Calculate the moles of a solute dissolved in a solution. You multiply the molarity by the number of osmoles that each solute produces. Osmolarity is the number of osmoles of solute per litre of solution. Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): To calculate the osmolarity. How To Calculate The Osmolality Of A Solution.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 How To Calculate The Osmolality Of A Solution Unless a solution is very concentrated, the osmolarity of a solution (c) can be calculated from its experimentally determined osmolality (m): Convert that number to osmoles per liter and add the osmolarity of each. An osmole is 1 mol of particles that contribute to the osmotic. Now calculate the molarity of the solution. To calculate the osmolarity of a solution,. How To Calculate The Osmolality Of A Solution.
From www.youtube.com
Calculate Molecular Weight from Osmotic Pressure for Nonideal Solution How To Calculate The Osmolality Of A Solution An osmole (osmol) is 1 mol of particles that. An osmole is 1 mol of particles that contribute to the osmotic. Steps required to measure the osmolarity of a solution: Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). Now calculate the molarity of the solution. Osmolarity is the. How To Calculate The Osmolality Of A Solution.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 How To Calculate The Osmolality Of A Solution To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Osmolarity is the number of osmoles of solute per litre of solution. An osmole (osmol) is 1 mol of particles that. An osmole is 1 mol of particles that contribute to the osmotic. Calculate the moles of a solute dissolved in. How To Calculate The Osmolality Of A Solution.
From www.numerade.com
SOLVED 6. Determine the molarity and osmolarity of the following How To Calculate The Osmolality Of A Solution To calculate the osmolarity of a solution, the first step is to convert the number to moles per liter. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity (osmol). An osmole (osmol) is 1 mol of particles that. Now calculate the molarity of the solution. Calculate the moles of a. How To Calculate The Osmolality Of A Solution.