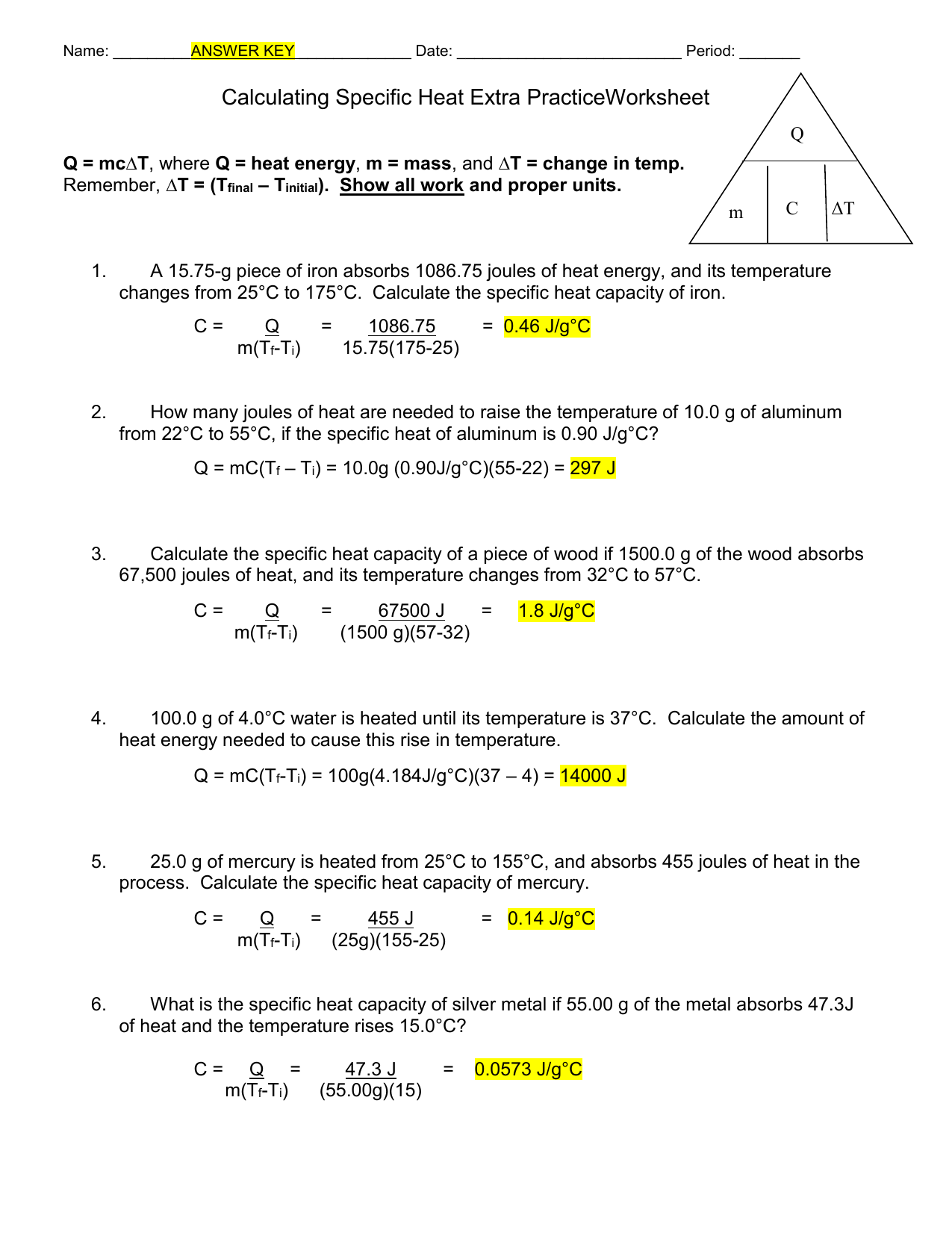
Specific Heat Capacity And Calorimetry Worksheet Answers . Specific heat and heat capacity worksheet. How much heat did this. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). Practice problems on heat capacity and specific heat. Show all work and proper. Specific heat capacity can be described as a substance's resistance to temperature changes. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Which substance has a greater specific heat. If the heat capacity of the calorimeter. Examples of how to determine, the heat, heat capacity, and change of temperature. Specific heat worksheet name (in ink): When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. How many grams of water can be heated from 20.0.
from lessonfullfrancie.z21.web.core.windows.net
Practice problems on heat capacity and specific heat. Show all work and proper. If the heat capacity of the calorimeter. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? How many grams of water can be heated from 20.0. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. How much heat did this. C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). Examples of how to determine, the heat, heat capacity, and change of temperature.
Specific Heat Worksheets With Answers
Specific Heat Capacity And Calorimetry Worksheet Answers The temperature of 335 g of water changed from 24.5oc to 26.4oc. Which substance has a greater specific heat. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Show all work and proper. C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Practice problems on heat capacity and specific heat. If the heat capacity of the calorimeter. Specific heat capacity can be described as a substance's resistance to temperature changes. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Specific heat worksheet name (in ink): How much heat did this. Examples of how to determine, the heat, heat capacity, and change of temperature. The temperature of 335 g of water changed from 24.5oc to 26.4oc. Specific heat and heat capacity worksheet. How many grams of water can be heated from 20.0.
From lessonmedianadeau.z21.web.core.windows.net
Specific Heat And Calorimetry Worksheet Specific Heat Capacity And Calorimetry Worksheet Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? The temperature of 335 g of water changed from 24.5oc to 26.4oc. Examples of how to determine, the heat, heat capacity, and change of temperature. Practice. Specific Heat Capacity And Calorimetry Worksheet Answers.
From worksheets.clipart-library.com
Free heat capacity worksheet, Download Free heat capacity worksheet png Specific Heat Capacity And Calorimetry Worksheet Answers Specific heat and heat capacity worksheet. C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). How much heat did this. Specific heat worksheet name (in ink): If the heat capacity of the calorimeter. How much energy is needed to change the temperature of 50.0 g of water by. Specific Heat Capacity And Calorimetry Worksheet Answers.
From worksheetmagicritter101.z21.web.core.windows.net
Specific Heat And Heat Capacity Worksheet Answers Specific Heat Capacity And Calorimetry Worksheet Answers How much heat did this. If the heat capacity of the calorimeter. Which substance has a greater specific heat. Specific heat worksheet name (in ink): Practice problems on heat capacity and specific heat. Show all work and proper. Specific heat capacity can be described as a substance's resistance to temperature changes. C = q/mat, where q = heat energy, m. Specific Heat Capacity And Calorimetry Worksheet Answers.
From learninglibraryralf.z13.web.core.windows.net
Specific Heat And Calorimetry Worksheet Specific Heat Capacity And Calorimetry Worksheet Answers The temperature of 335 g of water changed from 24.5oc to 26.4oc. Which substance has a greater specific heat. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Show all work and proper. 1) if 0.315 moles. Specific Heat Capacity And Calorimetry Worksheet Answers.
From worksheets.clipart-library.com
Calorimetry Worksheet Practice Problems and Solutions Specific Heat Capacity And Calorimetry Worksheet Answers When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Specific heat worksheet name (in ink): How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Which substance has a greater specific heat. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing. Specific Heat Capacity And Calorimetry Worksheet Answers.
From www.studocu.com
Heat Capacity Calorimetry Worksheet answers NAME_________________PER Specific Heat Capacity And Calorimetry Worksheet Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Practice problems on heat capacity and specific heat. How much heat did this. C = q/mat, where q = heat energy, m = mass, and. Specific Heat Capacity And Calorimetry Worksheet Answers.
From learningfullherman.z19.web.core.windows.net
Specific Heat Worksheet 2 Answer Key Specific Heat Capacity And Calorimetry Worksheet Answers Specific heat and heat capacity worksheet. Specific heat worksheet name (in ink): How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? The temperature of 335 g of water changed from 24.5oc to 26.4oc. How much heat did this. Show all work and proper. Which substance has a greater specific heat. = mc∆t, where. Specific Heat Capacity And Calorimetry Worksheet Answers.
From printablemagiccolin.z13.web.core.windows.net
Specific Heat And Calorimetry Worksheets Specific Heat Capacity And Calorimetry Worksheet Answers Specific heat and heat capacity worksheet. Specific heat capacity can be described as a substance's resistance to temperature changes. Specific heat worksheet name (in ink): If the heat capacity of the calorimeter. Examples of how to determine, the heat, heat capacity, and change of temperature. = mc∆t, where q = heat energy, m = mass, and ∆t = change in. Specific Heat Capacity And Calorimetry Worksheet Answers.
From www.liveworksheets.com
Specific heat capacity nferguson Live Worksheets Specific Heat Capacity And Calorimetry Worksheet Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? How much heat did this. Specific heat worksheet name (in ink): How many grams of water can be heated from 20.0. Specific heat capacity can be described as a substance's resistance to temperature changes. Practice problems on heat capacity and specific heat. C =. Specific Heat Capacity And Calorimetry Worksheet Answers.
From learningnovotyzy.z21.web.core.windows.net
Heat And Calorimetry Worksheets Specific Heat Capacity And Calorimetry Worksheet Answers Which substance has a greater specific heat. Examples of how to determine, the heat, heat capacity, and change of temperature. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? How many grams of water can be heated from 20.0. How much heat did this. = mc∆t, where q = heat energy, m =. Specific Heat Capacity And Calorimetry Worksheet Answers.
From obropolox.blogspot.com
40 specific heat capacity worksheet answers Worksheet Resource Specific Heat Capacity And Calorimetry Worksheet Answers C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? If the heat capacity of the calorimeter. Which substance has a greater specific heat. How many grams of water can be heated from. Specific Heat Capacity And Calorimetry Worksheet Answers.
From docslib.org
Specific Heat and Heat Capacity Worksheet DocsLib Specific Heat Capacity And Calorimetry Worksheet Answers When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. If the heat capacity of the. Specific Heat Capacity And Calorimetry Worksheet Answers.
From studyschoolburman.z21.web.core.windows.net
Heat And Calorimetry Worksheet Answer Key Specific Heat Capacity And Calorimetry Worksheet Answers How much heat did this. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. How many grams of water can be heated from 20.0. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. How much energy is needed to change the temperature of. Specific Heat Capacity And Calorimetry Worksheet Answers.
From www.pinterest.com
50 Calorimetry Worksheet Answer Key Chessmuseum Template Library Specific Heat Capacity And Calorimetry Worksheet Answers Examples of how to determine, the heat, heat capacity, and change of temperature. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Specific heat capacity can be described as a substance's resistance to temperature changes. How much heat did this. Specific heat worksheet name (in ink): The temperature of 335 g. Specific Heat Capacity And Calorimetry Worksheet Answers.
From www.studypool.com
SOLUTION 6chap heat capacity specific heat worksheet Studypool Specific Heat Capacity And Calorimetry Worksheet Answers If the heat capacity of the calorimeter. Examples of how to determine, the heat, heat capacity, and change of temperature. How much heat did this. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Specific heat and heat capacity worksheet. Specific heat capacity can be described as a substance's resistance to temperature. Specific Heat Capacity And Calorimetry Worksheet Answers.
From classmediaabeeiderdowns.z21.web.core.windows.net
Specific Heat And Calorimetry Worksheets Specific Heat Capacity And Calorimetry Worksheet Answers C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). Examples of how to determine, the heat, heat capacity, and change of temperature. If the heat capacity of the calorimeter. Practice problems on heat capacity and specific heat. How much heat did this. How many grams of water can. Specific Heat Capacity And Calorimetry Worksheet Answers.
From worksheets.clipart-library.com
Free specific heat and heat capacity worksheet, Download Free specific Specific Heat Capacity And Calorimetry Worksheet Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Practice problems on heat capacity and specific heat. Specific heat capacity can be described as a substance's resistance to temperature changes. Show all work and proper. Which. Specific Heat Capacity And Calorimetry Worksheet Answers.
From printablezoneklaudia.z19.web.core.windows.net
Specific Heat And Calorimetry Worksheets Specific Heat Capacity And Calorimetry Worksheet Answers Specific heat and heat capacity worksheet. Show all work and proper. Which substance has a greater specific heat. If the heat capacity of the calorimeter. Practice problems on heat capacity and specific heat. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Examples of how to determine, the heat, heat capacity, and. Specific Heat Capacity And Calorimetry Worksheet Answers.
From materialliboster.z19.web.core.windows.net
Heat Calculations Worksheet Answers Specific Heat Capacity And Calorimetry Worksheet Answers The temperature of 335 g of water changed from 24.5oc to 26.4oc. Practice problems on heat capacity and specific heat. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Specific heat capacity can be described as a substance's resistance to temperature changes. If the heat capacity of the calorimeter. Examples of how. Specific Heat Capacity And Calorimetry Worksheet Answers.
From worksheets.clipart-library.com
Free specific heat worksheet answers, Download Free specific heat Specific Heat Capacity And Calorimetry Worksheet Answers Which substance has a greater specific heat. How much heat did this. If the heat capacity of the calorimeter. Practice problems on heat capacity and specific heat. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Show all work and proper. = mc∆t, where q = heat energy, m = mass, and ∆t. Specific Heat Capacity And Calorimetry Worksheet Answers.
From www.onlineworksheet.my.id
Specific Heat Worksheet Answers Specific Heat Capacity And Calorimetry Worksheet Answers Examples of how to determine, the heat, heat capacity, and change of temperature. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. If the heat capacity of the calorimeter. Specific heat and heat capacity worksheet. Which substance has a greater specific heat. How many grams of water can be heated from. Specific Heat Capacity And Calorimetry Worksheet Answers.
From printablezoneklaudia.z19.web.core.windows.net
Specific Heat And Calorimetry Worksheets Specific Heat Capacity And Calorimetry Worksheet Answers Specific heat capacity can be described as a substance's resistance to temperature changes. How much heat did this. Show all work and proper. Specific heat and heat capacity worksheet. How many grams of water can be heated from 20.0. If the heat capacity of the calorimeter. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing. Specific Heat Capacity And Calorimetry Worksheet Answers.
From davida.davivienda.com
Specific Heat Calculations Worksheet Answers Printable Word Searches Specific Heat Capacity And Calorimetry Worksheet Answers How many grams of water can be heated from 20.0. Specific heat capacity can be described as a substance's resistance to temperature changes. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Specific heat and heat capacity worksheet. The temperature of 335 g of water changed from 24.5oc to 26.4oc. How. Specific Heat Capacity And Calorimetry Worksheet Answers.
From materiallibraryyan.z13.web.core.windows.net
Specific Heat And Calorimetry Worksheet Specific Heat Capacity And Calorimetry Worksheet Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Practice problems on heat capacity and specific heat. Specific heat and heat capacity worksheet. Show all work and proper. Specific heat capacity can be described as a substance's resistance to temperature changes. C = q/mat, where q = heat energy, m = mass, and. Specific Heat Capacity And Calorimetry Worksheet Answers.
From worksheetwheedler.z21.web.core.windows.net
Worksheets Introduction To Specific Heat Capacities Specific Heat Capacity And Calorimetry Worksheet Answers The temperature of 335 g of water changed from 24.5oc to 26.4oc. C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). Specific heat capacity can be described as a substance's resistance to temperature changes. How much energy is needed to change the temperature of 50.0 g of water. Specific Heat Capacity And Calorimetry Worksheet Answers.
From wordworksheet.com
Specific Heat Worksheet Answers Specific Heat Capacity And Calorimetry Worksheet Answers Show all work and proper. Practice problems on heat capacity and specific heat. Specific heat capacity can be described as a substance's resistance to temperature changes. Specific heat worksheet name (in ink): Specific heat and heat capacity worksheet. Which substance has a greater specific heat. C = q/mat, where q = heat energy, m = mass, and t = temperature. Specific Heat Capacity And Calorimetry Worksheet Answers.
From zipworksheet.com
Specific Heat Worksheet Answer Key Specific Heat Capacity And Calorimetry Worksheet Answers If the heat capacity of the calorimeter. Which substance has a greater specific heat. Specific heat capacity can be described as a substance's resistance to temperature changes. C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb. Specific Heat Capacity And Calorimetry Worksheet Answers.
From worksheets.clipart-library.com
Free specific heat worksheet with answers, Download Free specific heat Specific Heat Capacity And Calorimetry Worksheet Answers Specific heat capacity can be described as a substance's resistance to temperature changes. Show all work and proper. If the heat capacity of the calorimeter. The temperature of 335 g of water changed from 24.5oc to 26.4oc. How much heat did this. Examples of how to determine, the heat, heat capacity, and change of temperature. Specific heat and heat capacity. Specific Heat Capacity And Calorimetry Worksheet Answers.
From worksheets.clipart-library.com
Calculating Specific Heat Worksheet Worksheet Worksheets Library Specific Heat Capacity And Calorimetry Worksheet Answers C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. How many grams of water can be heated from 20.0. Practice problems on heat capacity and specific heat. How much energy. Specific Heat Capacity And Calorimetry Worksheet Answers.
From materialmediaonshore.z14.web.core.windows.net
Specific Heat And Calorimetry Worksheet Specific Heat Capacity And Calorimetry Worksheet Answers Specific heat capacity can be described as a substance's resistance to temperature changes. Show all work and proper. Specific heat worksheet name (in ink): The temperature of 335 g of water changed from 24.5oc to 26.4oc. If the heat capacity of the calorimeter. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? When. Specific Heat Capacity And Calorimetry Worksheet Answers.
From learningfullherman.z19.web.core.windows.net
Specific Heat And Heat Capacity Worksheet Specific Heat Capacity And Calorimetry Worksheet Answers Specific heat and heat capacity worksheet. Examples of how to determine, the heat, heat capacity, and change of temperature. The temperature of 335 g of water changed from 24.5oc to 26.4oc. Specific heat capacity can be described as a substance's resistance to temperature changes. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48. Specific Heat Capacity And Calorimetry Worksheet Answers.
From manualfixfaber.z19.web.core.windows.net
Calculating Specific Heat Worksheet Answers Specific Heat Capacity And Calorimetry Worksheet Answers Practice problems on heat capacity and specific heat. C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). How many grams of water can be heated from 20.0. Show all work and proper. Specific heat capacity can be described as a substance's resistance to temperature changes. 1) if 0.315. Specific Heat Capacity And Calorimetry Worksheet Answers.
From www.worksheeto.com
10 Science Worksheets On Heat / Specific Heat Capacity And Calorimetry Worksheet Answers The temperature of 335 g of water changed from 24.5oc to 26.4oc. C = q/mat, where q = heat energy, m = mass, and t = temperature remember, at = (tfinal — tinitial). Practice problems on heat capacity and specific heat. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. If the. Specific Heat Capacity And Calorimetry Worksheet Answers.
From lessonfullfrancie.z21.web.core.windows.net
Specific Heat Worksheets With Answers Specific Heat Capacity And Calorimetry Worksheet Answers Specific heat and heat capacity worksheet. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Practice problems on heat capacity and specific heat. = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter. Specific Heat Capacity And Calorimetry Worksheet Answers.
From materiallibmuller.z19.web.core.windows.net
Specific Heat Practice Worksheet Answers Specific Heat Capacity And Calorimetry Worksheet Answers Show all work and proper. Which substance has a greater specific heat. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water,. Specific heat and heat capacity worksheet. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. How much heat did this. How many. Specific Heat Capacity And Calorimetry Worksheet Answers.