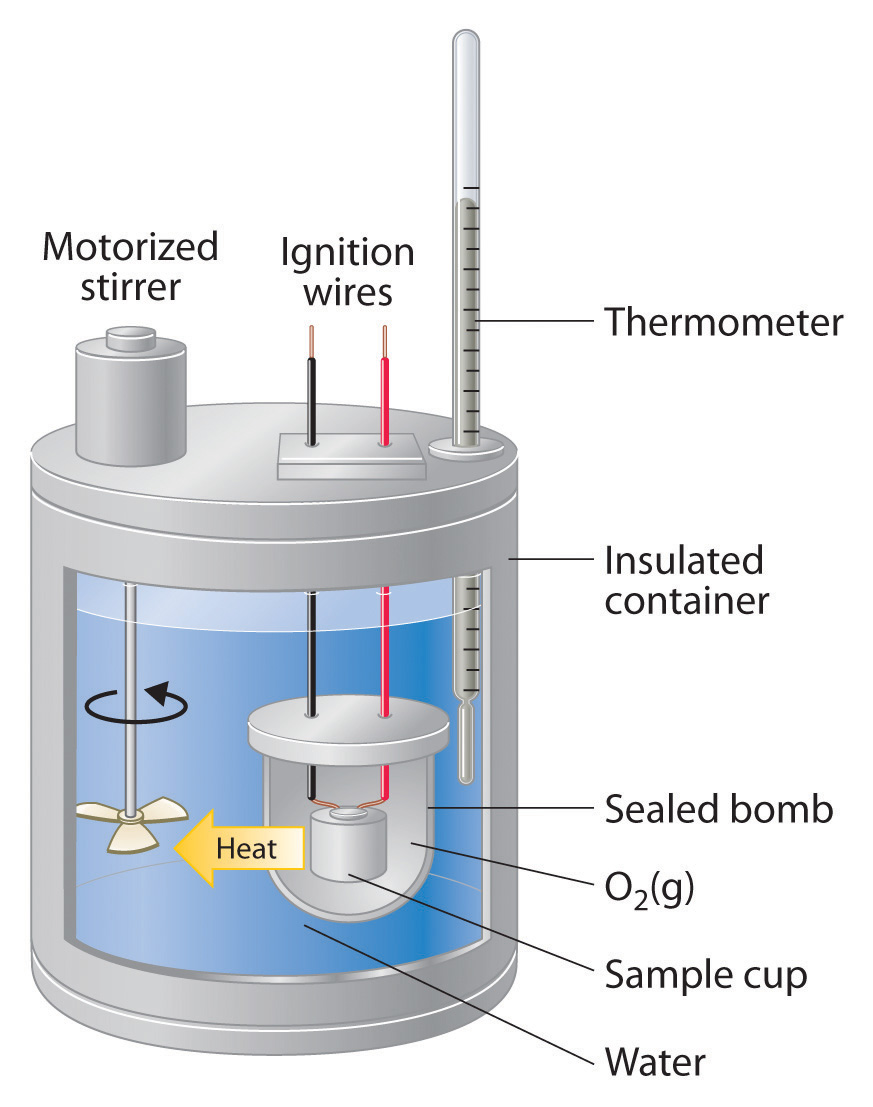
Calorimetry Hand Warmer Lab . The investigation begins with an introductory activity to become familiar with the principles of calorimetry and heat of solution calculations. • measure the temperature change of the dissolution of a variety of salts in water. Designing an economical hand warmer. • calculate the calorimeter constant. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. The purpose of this investigation is to investigate energy transfer using calorimetry. Building a better hand warmer. The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. • calculate the heat of. By pressing a button in a pouch,. Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as.
from sites.google.com
• calculate the calorimeter constant. Designing an economical hand warmer. • measure the temperature change of the dissolution of a variety of salts in water. Building a better hand warmer. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as. The purpose of this investigation is to investigate energy transfer using calorimetry. Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. The investigation begins with an introductory activity to become familiar with the principles of calorimetry and heat of solution calculations.
Calorimetry Preliminary HSC Chemistry
Calorimetry Hand Warmer Lab The investigation begins with an introductory activity to become familiar with the principles of calorimetry and heat of solution calculations. By pressing a button in a pouch,. Building a better hand warmer. Designing an economical hand warmer. • calculate the calorimeter constant. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as. • calculate the heat of. Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. The investigation begins with an introductory activity to become familiar with the principles of calorimetry and heat of solution calculations. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. • measure the temperature change of the dissolution of a variety of salts in water. The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. The purpose of this investigation is to investigate energy transfer using calorimetry.
From www.youtube.com
Hot Hands (Hand Warmer) Lab Experiment YouTube Calorimetry Hand Warmer Lab By pressing a button in a pouch,. • calculate the calorimeter constant. • calculate the heat of. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. The ideal hand warmer increases in temperature. Calorimetry Hand Warmer Lab.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Hand Warmer Lab Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. • measure the temperature change of the dissolution of a variety of salts in water. Designing an economical hand warmer. Building a better hand warmer. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible,. Calorimetry Hand Warmer Lab.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Hand Warmer Lab The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. • calculate the heat of. • measure the temperature change of the dissolution of a variety of salts in water. Designing an economical hand warmer. The. Calorimetry Hand Warmer Lab.
From www.youtube.com
Designing a Hand Warmer Lab YouTube Calorimetry Hand Warmer Lab Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. Designing an economical hand warmer. • measure the temperature change of the dissolution of a variety of salts in water. The purpose of this. Calorimetry Hand Warmer Lab.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Hand Warmer Lab Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. Building a better hand warmer. • measure the temperature change of the dissolution of a variety of salts in water. The purpose of this investigation is to investigate energy transfer using calorimetry. Designing an economical hand warmer. Students use a temperature sensor. Calorimetry Hand Warmer Lab.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry Hand Warmer Lab Building a better hand warmer. Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. • calculate the heat of. The purpose of this investigation is to investigate energy transfer using calorimetry. • calculate the calorimeter constant. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly. Calorimetry Hand Warmer Lab.
From slideplayer.com
Calorimetry Chapter ppt download Calorimetry Hand Warmer Lab • measure the temperature change of the dissolution of a variety of salts in water. Designing an economical hand warmer. By pressing a button in a pouch,. • calculate the heat of. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as.. Calorimetry Hand Warmer Lab.
From wisc.pb.unizin.org
Day 27 Thermochemistry and Enthalpy Chemistry 109, Fall 2020 Calorimetry Hand Warmer Lab • calculate the calorimeter constant. Building a better hand warmer. Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. Designing an economical hand warmer. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little. Calorimetry Hand Warmer Lab.
From sciencenotes.org
Hand Warmer Chemistry Easy Chemical Hot Packs Calorimetry Hand Warmer Lab Building a better hand warmer. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as. • calculate the calorimeter constant. • calculate the heat of. The purpose of this investigation is to investigate energy transfer using calorimetry. Hand warmers provide a unique. Calorimetry Hand Warmer Lab.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab Calorimetry Hand Warmer Lab The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. • calculate the heat of. Building a better hand warmer. • calculate the calorimeter constant. The investigation begins with an introductory activity to become familiar with. Calorimetry Hand Warmer Lab.
From slideplayer.com
CHEMISTRY Thermochemistry Chapter 5 ppt download Calorimetry Hand Warmer Lab • calculate the calorimeter constant. The investigation begins with an introductory activity to become familiar with the principles of calorimetry and heat of solution calculations. By pressing a button in a pouch,. The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible. Calorimetry Hand Warmer Lab.
From saylordotorg.github.io
Calorimetry Calorimetry Hand Warmer Lab • measure the temperature change of the dissolution of a variety of salts in water. By pressing a button in a pouch,. • calculate the heat of. Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. The investigation begins with an introductory activity to become familiar with the principles of calorimetry. Calorimetry Hand Warmer Lab.
From www.scribd.com
Hand Out 1.5 Specific Heat Calorimetry Lab PDF Heat Calorimetry Calorimetry Hand Warmer Lab • measure the temperature change of the dissolution of a variety of salts in water. Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. The purpose of this investigation is to investigate energy transfer using calorimetry. • calculate the heat of. • calculate the calorimeter constant. Designing an economical hand warmer.. Calorimetry Hand Warmer Lab.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Hand Warmer Lab The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. Designing an economical hand warmer. • calculate the heat of. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as. Calorimetry Hand Warmer Lab.
From www.youtube.com
Designing a Hand Warmer Lab YouTube Calorimetry Hand Warmer Lab By pressing a button in a pouch,. Designing an economical hand warmer. The investigation begins with an introductory activity to become familiar with the principles of calorimetry and heat of solution calculations. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as.. Calorimetry Hand Warmer Lab.
From www.youtube.com
AP Chemistry Designing a Hand Warmer Lab YouTube Calorimetry Hand Warmer Lab The investigation begins with an introductory activity to become familiar with the principles of calorimetry and heat of solution calculations. The purpose of this investigation is to investigate energy transfer using calorimetry. Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. Building a better hand warmer. The ideal hand warmer increases. Calorimetry Hand Warmer Lab.
From www.thinkswap.com
Calorimetry Handwarmer Lab Report Science Grade 10 Dogwood Calorimetry Hand Warmer Lab The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. The purpose of this investigation is to investigate energy transfer using calorimetry. • calculate the calorimeter constant. The investigation begins with an introductory activity to become. Calorimetry Hand Warmer Lab.
From www.youtube.com
Unit 8 Food Calorimetry Lab YouTube Calorimetry Hand Warmer Lab Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. • measure the temperature change of the dissolution of a variety of salts in water. The purpose of this investigation is to investigate energy transfer using calorimetry. • calculate the calorimeter constant. The investigation begins with an introductory activity to become familiar. Calorimetry Hand Warmer Lab.
From isabelladp.weebly.com
Calorimetry Lab Belle's Digital Portfolio Calorimetry Hand Warmer Lab • calculate the calorimeter constant. The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of. Calorimetry Hand Warmer Lab.
From www.youtube.com
Ap Chemistry Hand Warmer Lab YouTube Calorimetry Hand Warmer Lab The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as. By pressing a button in a pouch,. Designing an economical hand warmer. The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of. Calorimetry Hand Warmer Lab.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Hand Warmer Lab The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as. Calorimetry Hand Warmer Lab.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Hand Warmer Lab The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. The investigation begins with an introductory activity to. Calorimetry Hand Warmer Lab.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Hand Warmer Lab • calculate the calorimeter constant. The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. Building a better hand warmer. The investigation begins with an introductory activity to become familiar with the principles of calorimetry and. Calorimetry Hand Warmer Lab.
From www.showme.com
Calorimeter Calibration for Hand Warmer Lab Science, Chemical Calorimetry Hand Warmer Lab The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. Building a better hand warmer. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of. Calorimetry Hand Warmer Lab.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Hand Warmer Lab Designing an economical hand warmer. The investigation begins with an introductory activity to become familiar with the principles of calorimetry and heat of solution calculations. Building a better hand warmer. By pressing a button in a pouch,. Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. • calculate the calorimeter constant.. Calorimetry Hand Warmer Lab.
From www.studocu.com
Calorimetry Lab Lab The Hand Warmer Design (Calorimetry Calorimetry Hand Warmer Lab Building a better hand warmer. Designing an economical hand warmer. By pressing a button in a pouch,. The purpose of this investigation is to investigate energy transfer using calorimetry. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. The investigation begins with an introductory activity to become familiar with the principles. Calorimetry Hand Warmer Lab.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Calorimetry Hand Warmer Lab Building a better hand warmer. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as. The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as. Calorimetry Hand Warmer Lab.
From chapter4skinandbodymembranes.blogspot.com
The Hand Warmer Design Challenge Pre Lab Answers Hand Warmer Lab Docx Calorimetry Hand Warmer Lab • calculate the calorimeter constant. Designing an economical hand warmer. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as. By pressing a button in a pouch,. The purpose of this investigation is to investigate energy transfer using calorimetry. The ideal hand. Calorimetry Hand Warmer Lab.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Calorimetry Hand Warmer Lab The purpose of this investigation is to investigate energy transfer using calorimetry. • calculate the heat of. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs. Calorimetry Hand Warmer Lab.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry Hand Warmer Lab • measure the temperature change of the dissolution of a variety of salts in water. • calculate the calorimeter constant. Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. Designing an economical hand warmer. The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has. Calorimetry Hand Warmer Lab.
From courses.lumenlearning.com
Calorimetry Chemistry Calorimetry Hand Warmer Lab The ideal hand warmer increases in temperature by 20°c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as possible to make, and uses chemicals that. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as. Calorimetry Hand Warmer Lab.
From www.avantorsciences.com
Ward's® AP Chemistry Investigation 12 Calorimetry How Does a Hand Calorimetry Hand Warmer Lab Students use a temperature sensor to determine the heat of solution of a number of ionic compounds and. By pressing a button in a pouch,. • calculate the calorimeter constant. Building a better hand warmer. The investigation begins with an introductory activity to become familiar with the principles of calorimetry and heat of solution calculations. The ideal hand warmer increases. Calorimetry Hand Warmer Lab.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimetry Hand Warmer Lab • calculate the heat of. The investigation begins with an introductory activity to become familiar with the principles of calorimetry and heat of solution calculations. Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. The purpose of this investigation is to investigate energy transfer using calorimetry. Building a better hand warmer.. Calorimetry Hand Warmer Lab.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Hand Warmer Lab Hand warmers provide a unique and fun way to study the chemistry of crystal formation and heat generation. • calculate the calorimeter constant. By pressing a button in a pouch,. The purpose of this investigation is to investigate energy transfer using calorimetry. Designing an economical hand warmer. The ideal hand warmer increases in temperature by 20 °c (but no more). Calorimetry Hand Warmer Lab.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Hand Warmer Lab • calculate the calorimeter constant. Designing an economical hand warmer. By pressing a button in a pouch,. The purpose of this investigation is to investigate energy transfer using calorimetry. The ideal hand warmer increases in temperature by 20 °c (but no more) as quickly as possible, has a volume of about 50 ml, costs as little as. Building a better. Calorimetry Hand Warmer Lab.