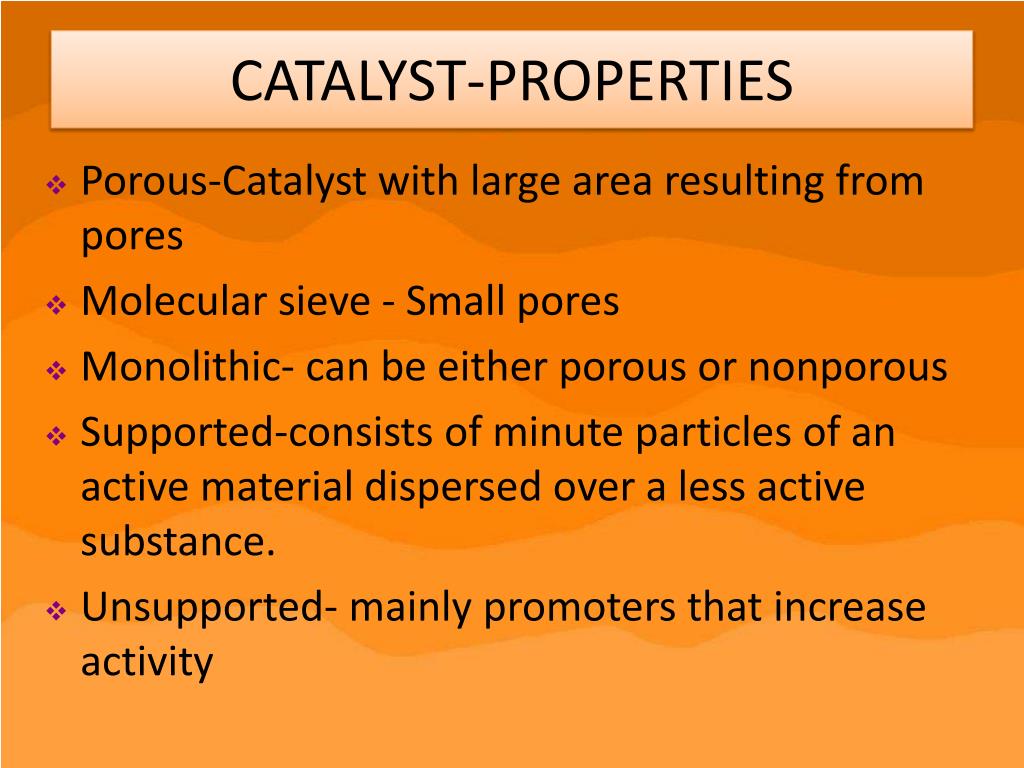
Catalyst Properties In Reactions . Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Reaction diagrams for catalyzed reactions. One without a catalyst and one with a. The two reaction diagrams here represent the same reaction: Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. In heterogeneous catalysis, catalysts provide a surface to. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts are not consumed by a reaction.
from www.slideserve.com
Therefore they are crucially important in chemical industry, exhaust gas treatment and. The two reaction diagrams here represent the same reaction: What are catalysts, and how do they work in terms altering the parameters of a reaction? One without a catalyst and one with a. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Reaction diagrams for catalyzed reactions. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions.
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint
Catalyst Properties In Reactions A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Reaction diagrams for catalyzed reactions. The two reaction diagrams here represent the same reaction: Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Therefore they are crucially important in chemical industry, exhaust gas treatment and. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. One without a catalyst and one with a. Catalysts are not consumed by a reaction. In heterogeneous catalysis, catalysts provide a surface to. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction.
From www.slideshare.net
Catalysis Catalyst Properties In Reactions Reaction diagrams for catalyzed reactions. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts are not consumed by a reaction. The two reaction diagrams here represent the same reaction: Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalysts reduce the activation energy of reactions and enhance the rate. Catalyst Properties In Reactions.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Properties In Reactions A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. The two reaction diagrams here represent the same reaction: Catalysts are not consumed by a reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? Therefore they are crucially important in chemical industry, exhaust gas treatment. Catalyst Properties In Reactions.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Properties In Reactions Reaction diagrams for catalyzed reactions. Therefore they are crucially important in chemical industry, exhaust gas treatment and. The two reaction diagrams here represent the same reaction: One without a catalyst and one with a. Catalysts are not consumed by a reaction. In heterogeneous catalysis, catalysts provide a surface to. Catalysts reduce the activation energy of reactions and enhance the rate. Catalyst Properties In Reactions.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Properties In Reactions A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Therefore they are crucially important in chemical industry, exhaust gas treatment and. Reaction diagrams for catalyzed reactions. Catalysis, the modification of the rate of. Catalyst Properties In Reactions.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst Properties In Reactions A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. Reaction diagrams for catalyzed reactions. In heterogeneous catalysis, catalysts provide a surface to. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts reduce the activation energy of reactions. Catalyst Properties In Reactions.
From www.onlinebiologynotes.com
Enzymes Properties and Mechanism of enzyme action Online Biology Notes Catalyst Properties In Reactions Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. What are catalysts, and how do they. Catalyst Properties In Reactions.
From www.nagwa.com
Question Video Identifying the Reason Why Catalysts Are Used in Catalyst Properties In Reactions Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? In heterogeneous catalysis, catalysts provide a surface to. Therefore they are crucially important in chemical industry, exhaust gas treatment and. Reaction. Catalyst Properties In Reactions.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst Properties In Reactions Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. The two reaction diagrams here represent the same reaction: Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are not consumed. Catalyst Properties In Reactions.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalyst Properties In Reactions In heterogeneous catalysis, catalysts provide a surface to. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. One without a catalyst and one with a. Reaction diagrams for catalyzed reactions. Therefore they are crucially important in chemical industry, exhaust. Catalyst Properties In Reactions.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation Catalyst Properties In Reactions Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts allow a reaction to proceed via a pathway that has. Catalyst Properties In Reactions.
From 2012books.lardbucket.org
Catalysis Catalyst Properties In Reactions The two reaction diagrams here represent the same reaction: In heterogeneous catalysis, catalysts provide a surface to. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst lowers the activation energy of a reaction, making it more thermodynamically. Catalyst Properties In Reactions.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Properties In Reactions The two reaction diagrams here represent the same reaction: In heterogeneous catalysis, catalysts provide a surface to. Catalysts are not consumed by a reaction. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalyst, in chemistry, any substance that increases the rate of. Catalyst Properties In Reactions.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalyst Properties In Reactions In heterogeneous catalysis, catalysts provide a surface to. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts are not consumed by a reaction. Reaction diagrams for catalyzed reactions. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. The two reaction diagrams here represent the. Catalyst Properties In Reactions.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation ID591293 Catalyst Properties In Reactions Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. One without a catalyst and one with a. Reaction diagrams for catalyzed reactions. Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalysts are not consumed by. Catalyst Properties In Reactions.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Catalyst Properties In Reactions Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? Therefore they are crucially important in chemical industry, exhaust gas treatment. Catalyst Properties In Reactions.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Properties In Reactions Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. One without a catalyst and one with a. The two reaction diagrams here represent the same reaction: A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. Catalysts reduce the activation energy of reactions and enhance the. Catalyst Properties In Reactions.
From www.pinterest.com
Preparation of Alkane all methods and catalyst in one page. Biology Catalyst Properties In Reactions A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. In heterogeneous catalysis, catalysts provide a surface to. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Therefore they are crucially important in chemical industry, exhaust gas treatment and. The two reaction diagrams here represent the same reaction:. Catalyst Properties In Reactions.
From www.researchgate.net
Classification and summary of different catalysts. Download Catalyst Properties In Reactions Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Catalysts are not consumed by a reaction. Therefore they are crucially important in chemical industry, exhaust gas treatment and. Reaction diagrams for catalyzed reactions. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. The two reaction. Catalyst Properties In Reactions.
From www.youtube.com
Practice Identifying Catalysts and Intermediates YouTube Catalyst Properties In Reactions Reaction diagrams for catalyzed reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. Catalysts are not consumed by a reaction. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. In. Catalyst Properties In Reactions.
From slidetodoc.com
Heterogeneous Catalysis Solid State Physics 141 A Dohyung Catalyst Properties In Reactions Therefore they are crucially important in chemical industry, exhaust gas treatment and. Reaction diagrams for catalyzed reactions. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are not consumed by a reaction. Catalysts allow a reaction. Catalyst Properties In Reactions.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalyst Properties In Reactions Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts are not consumed by a reaction. Reaction diagrams for catalyzed reactions. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalyst,. Catalyst Properties In Reactions.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Properties In Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Reaction diagrams for catalyzed reactions. The two reaction diagrams here represent the same reaction: One without a catalyst and one with a. Catalyst, in chemistry,. Catalyst Properties In Reactions.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalyst Properties In Reactions Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. One without a catalyst and one with a. The two reaction diagrams here represent the same reaction: What are catalysts, and how do. Catalyst Properties In Reactions.
From www.slideserve.com
PPT CATALYSTS Sammy Tran PowerPoint Presentation ID443339 Catalyst Properties In Reactions Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to. One without a catalyst and one with a. A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. Reaction diagrams for catalyzed reactions. Catalysis, the modification. Catalyst Properties In Reactions.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Catalyst Properties In Reactions A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts are not consumed by a reaction. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a. Catalyst Properties In Reactions.
From www.researchgate.net
Properties of reactants, products, and catalysts used in the Catalyst Properties In Reactions Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Reaction diagrams for catalyzed reactions. A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. One without a catalyst and one with. Catalyst Properties In Reactions.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Properties In Reactions One without a catalyst and one with a. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts allow a. Catalyst Properties In Reactions.
From www.researchgate.net
Commonly Used Types of Catalysts and Their Range of Use Download Catalyst Properties In Reactions A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. What are catalysts, and how do they work in terms altering the parameters of a reaction? In heterogeneous catalysis, catalysts provide a surface to. Catalysts are not consumed by. Catalyst Properties In Reactions.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Properties In Reactions One without a catalyst and one with a. Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. In heterogeneous catalysis, catalysts provide a surface to. Catalyst, in chemistry, any substance that increases the rate. Catalyst Properties In Reactions.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Properties In Reactions Catalysts are not consumed by a reaction. In heterogeneous catalysis, catalysts provide a surface to. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Therefore they are crucially important in chemical industry, exhaust gas treatment and. One without a catalyst and one with a. A catalyst lowers the activation energy of a reaction, making it. Catalyst Properties In Reactions.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Properties In Reactions Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower. Catalyst Properties In Reactions.
From www.slideserve.com
PPT Heterogeneous Catalysis & Solid State Physics PowerPoint Catalyst Properties In Reactions In heterogeneous catalysis, catalysts provide a surface to. One without a catalyst and one with a. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis, the modification of the rate of a chemical. Catalyst Properties In Reactions.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Properties In Reactions Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. In heterogeneous catalysis, catalysts provide a surface to. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst lowers the activation energy of a reaction, making it more thermodynamically favorable, and thus faster. One without a catalyst and. Catalyst Properties In Reactions.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Properties In Reactions In heterogeneous catalysis, catalysts provide a surface to. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. One without a catalyst and one with a. Therefore they are crucially important in chemical industry, exhaust gas treatment and. What are catalysts, and how do they work in terms altering the parameters of a reaction? The two. Catalyst Properties In Reactions.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Catalyst Properties In Reactions One without a catalyst and one with a. Catalysts are not consumed by a reaction. Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Therefore they are crucially important in chemical industry, exhaust gas treatment and. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not. Catalyst Properties In Reactions.