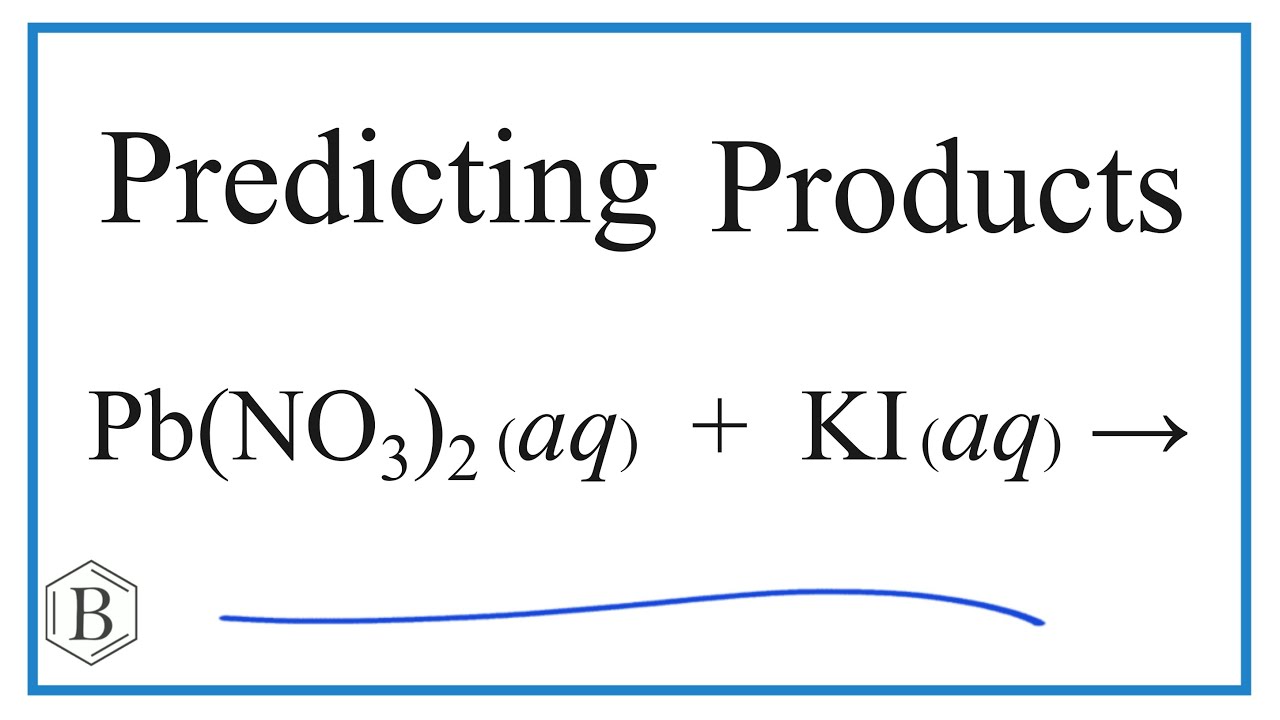
Lead (Ii) Nitrate + Potassium Iodide Reaction Type . Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. No when these two compounds react they will break into the. When you add lead nitrate to potassium iodide, the particles combine and create two new compounds: A yellow solid called lead iodide and a white solid called potassium nitrate. Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide.
from www.youtube.com
No when these two compounds react they will break into the. When you add lead nitrate to potassium iodide, the particles combine and create two new compounds: Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. A yellow solid called lead iodide and a white solid called potassium nitrate. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki.
Predict the Products of the Reaction for Pb(NO3)2 + KI Lead(II
Lead (Ii) Nitrate + Potassium Iodide Reaction Type A yellow solid called lead iodide and a white solid called potassium nitrate. When you add lead nitrate to potassium iodide, the particles combine and create two new compounds: No when these two compounds react they will break into the. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). A yellow solid called lead iodide and a white solid called potassium nitrate. Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide.
From www.youtube.com
Demonstration Precipitation Reaction of Potassium Iodide and Lead Lead (Ii) Nitrate + Potassium Iodide Reaction Type A yellow solid called lead iodide and a white solid called potassium nitrate. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Reactions of lead (ii) nitrate and. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From yazmingokefoster.blogspot.com
Particle Diagram of Lead Nitrate and Potassium Iodide Lead (Ii) Nitrate + Potassium Iodide Reaction Type No when these two compounds react they will break into the. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Reactions of lead (ii). Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From slideplayer.com
Types of Reactions. ppt download Lead (Ii) Nitrate + Potassium Iodide Reaction Type Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.youtube.com
007 Potassium Iodide + Lead (II) Nitrate = Lead (II) Iodide Lead (Ii) Nitrate + Potassium Iodide Reaction Type Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. A. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.studypool.com
SOLUTION Reaction of potassium iodide with lead nitrate Studypool Lead (Ii) Nitrate + Potassium Iodide Reaction Type Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. A yellow solid called lead iodide and a white solid called potassium nitrate. When you add lead nitrate to potassium iodide, the particles combine and create two new compounds: No when these two compounds react they will break into the. Formula of lead nitrate is pbno3. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.youtube.com
Potassium Iodide & Lead II Nitrate reaction YouTube Lead (Ii) Nitrate + Potassium Iodide Reaction Type Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. No when these two compounds react they will break into the. Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. A yellow solid called lead iodide and a white solid called potassium nitrate. Use this demonstration. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.youtube.com
Predict the Products of the Reaction for Pb(NO3)2 + KI Lead(II Lead (Ii) Nitrate + Potassium Iodide Reaction Type Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. A yellow solid called lead iodide and a white solid called potassium nitrate. No when these two compounds react they will break into the. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Lead(ii) nitrate (pb. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation ID5319064 Lead (Ii) Nitrate + Potassium Iodide Reaction Type A yellow solid called lead iodide and a white solid called potassium nitrate. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. No when these two compounds react they. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.numerade.com
SOLVED If you dissolve lead (II) nitrate and potassium iodide in water Lead (Ii) Nitrate + Potassium Iodide Reaction Type A yellow solid called lead iodide and a white solid called potassium nitrate. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Reactions of. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.compoundchem.com
Chemical Reactions Lead Iodide & 'Golden Rain' Compound Interest Lead (Ii) Nitrate + Potassium Iodide Reaction Type Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. When you add lead nitrate to potassium iodide, the particles combine and create two new compounds: Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.numerade.com
SOLVED Solutions of lead (II) nitrate and potassium iodide are mixed Lead (Ii) Nitrate + Potassium Iodide Reaction Type Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From slideplayer.com
Types of Chemical Reactions ppt download Lead (Ii) Nitrate + Potassium Iodide Reaction Type Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. A yellow solid called lead iodide and a white solid called potassium nitrate. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. No when. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From slideplayer.com
Chemical Reactions. ppt download Lead (Ii) Nitrate + Potassium Iodide Reaction Type No when these two compounds react they will break into the. Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.youtube.com
Potassium Iodide and Lead Nitrate Yellow PPT Double Displacement Lead (Ii) Nitrate + Potassium Iodide Reaction Type Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. No when these two compounds react they will break into the. Lead(ii) nitrate (pb (no. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.youtube.com
The Reaction Between Lead (II) Nitrate and Potassium Iodide YouTube Lead (Ii) Nitrate + Potassium Iodide Reaction Type No when these two compounds react they will break into the. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. When you add lead nitrate to potassium iodide, the particles combine and create two new compounds: A yellow solid called lead iodide and. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.youtube.com
Experiment on Lead nitrate and Potassium iodide I Class 10 Chemistry Lead (Ii) Nitrate + Potassium Iodide Reaction Type Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). A yellow solid called lead iodide and a white solid called potassium nitrate. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.youtube.com
REACTION OF LEAD NITRATE WITH POTASSIUM IODIDE YouTube Lead (Ii) Nitrate + Potassium Iodide Reaction Type A yellow solid called lead iodide and a white solid called potassium nitrate. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. No when these two compounds react they will break into the. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2). Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.chegg.com
Solved The precipitation reaction of lead(II) nitrate with Lead (Ii) Nitrate + Potassium Iodide Reaction Type Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). When you add lead nitrate to potassium iodide, the particles combine and create two new compounds: Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. No when these two compounds react they will. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.teachoo.com
Double Displacement Reaction Definition, Examples, Types Teachoo Lead (Ii) Nitrate + Potassium Iodide Reaction Type Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). No when these two compounds react they will break into the. Use this demonstration with kit list and safety instructions to prove that two. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.slideshare.net
Chemistry Lead (Ii) Nitrate + Potassium Iodide Reaction Type No when these two compounds react they will break into the. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. When you add lead. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Lead (Ii) Nitrate + Potassium Iodide Reaction Type No when these two compounds react they will break into the. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. Use this demonstration with kit list and safety instructions to prove that two. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.youtube.com
Double replacement reaction of lead (II) nitrate with potassium iodide Lead (Ii) Nitrate + Potassium Iodide Reaction Type When you add lead nitrate to potassium iodide, the particles combine and create two new compounds: Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. No when these two compounds react they will break into the. Lead(ii) nitrate (pb (no 3) 2) interacts. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.slideserve.com
PPT Types of Chemical Reactions PowerPoint Presentation, free Lead (Ii) Nitrate + Potassium Iodide Reaction Type Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. No when these two compounds react they will break into the. When you add lead nitrate to potassium iodide,. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From slideplayer.com
Introduction to Chemistry Chapter ppt download Lead (Ii) Nitrate + Potassium Iodide Reaction Type Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Lead (Ii) Nitrate + Potassium Iodide Reaction Type A yellow solid called lead iodide and a white solid called potassium nitrate. No when these two compounds react they will break into the. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Use this demonstration with kit list and safety instructions to prove that two solids can react together,. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From slideplayer.com
6 Basic Types of Reactions ppt download Lead (Ii) Nitrate + Potassium Iodide Reaction Type Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. A yellow solid called lead iodide and a white solid called potassium nitrate. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Lead(ii) nitrate. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.youtube.com
Lead Nitrate and Potassium Iodide Reaction ChemistryBimistry Labs Lead (Ii) Nitrate + Potassium Iodide Reaction Type No when these two compounds react they will break into the. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. When you add lead nitrate to potassium iodide, the particles combine and create two new compounds: Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid.. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.youtube.com
Precipitation Reaction Potassium Iodide KI & Lead (II) Nitrate Pb(NO3)2 Lead (Ii) Nitrate + Potassium Iodide Reaction Type Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. No when these two compounds react they will break into the. Formula of lead nitrate is pbno3 p b n. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Lead (Ii) Nitrate + Potassium Iodide Reaction Type Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. No when these two compounds react they will break into the. A yellow solid called lead iodide and a white solid called potassium nitrate. Lead(ii) nitrate (pb. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From picklifestyles.blogspot.com
Lead II Nitrate Reaction With Potassium Iodide Pb(NO3)2 Lifestyle News Lead (Ii) Nitrate + Potassium Iodide Reaction Type Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). No when these two compounds react they will break into the. A yellow solid called lead iodide and a. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.scribd.com
AP Chemistry I Chemical Reactions Lab Reaction 1 Lead (II) Nitrate Lead (Ii) Nitrate + Potassium Iodide Reaction Type Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. No when these two compounds react they will break into the. Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. Formula of lead nitrate is pbno3 p b n. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From slideplayer.com
Types of Chemical Reactions Single and Double Displacement ppt download Lead (Ii) Nitrate + Potassium Iodide Reaction Type Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.toppr.com
\"Reaction of potassium iodide solution with lead nitrate solution Lead (Ii) Nitrate + Potassium Iodide Reaction Type Reactions of lead (ii) nitrate and potassium iodideinitial appearance:clear liquid (lead) yellow transparent liquid. No when these two compounds react they will break into the. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Formula of lead nitrate is pbno3 p b n o 3 and the. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.youtube.com
Lead (II) nitrate solution reacts with potassium iodide solution. YouTube Lead (Ii) Nitrate + Potassium Iodide Reaction Type Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Formula of lead nitrate is pbno3 p b n o 3 and the formula of potassium iodide is ki. A yellow solid called lead iodide and a white solid called potassium nitrate. No when. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.
From www.youtube.com
Reaction of Lead Nitrate with Potassium Iodide YouTube Lead (Ii) Nitrate + Potassium Iodide Reaction Type A yellow solid called lead iodide and a white solid called potassium nitrate. When you add lead nitrate to potassium iodide, the particles combine and create two new compounds: No when these two compounds react they will break into the. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate. Lead (Ii) Nitrate + Potassium Iodide Reaction Type.