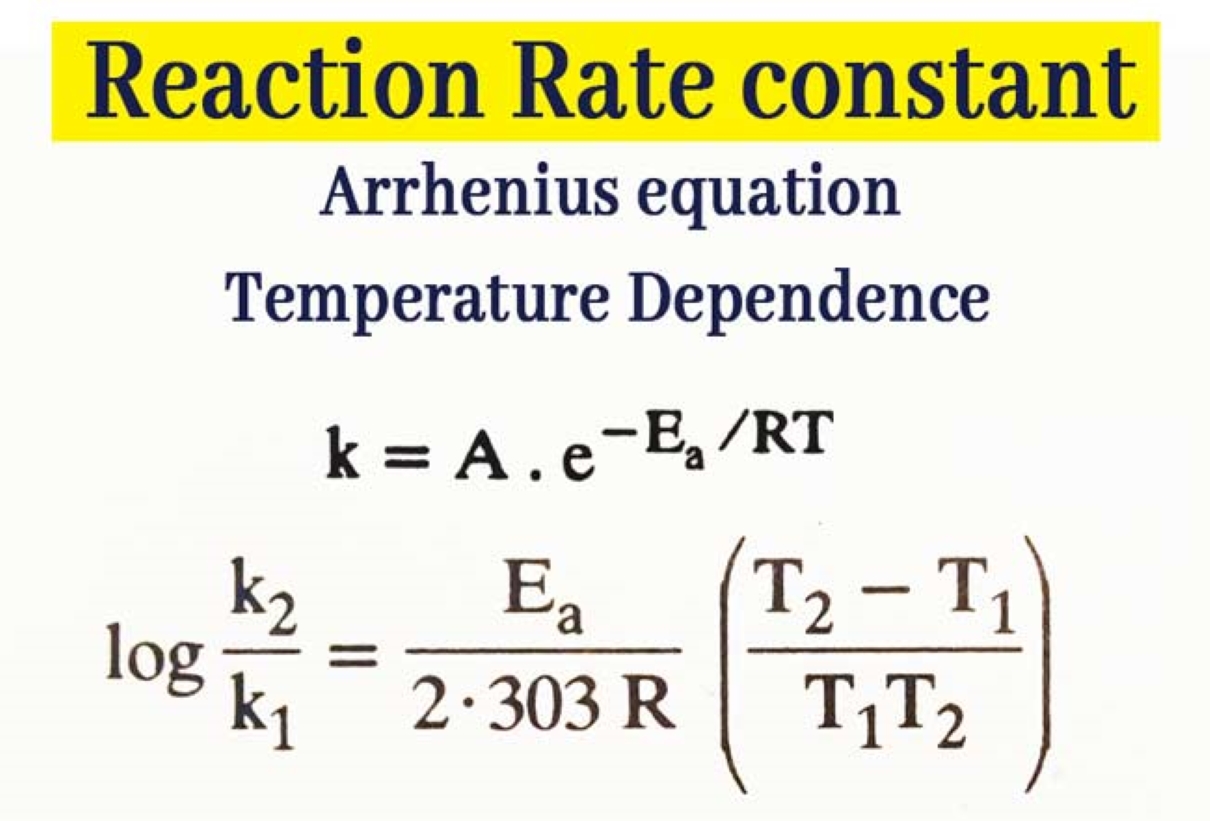
Rate Constant Arrhenius Definition . The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress. The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. If the rate constant doubles, for example,. It is also known as the reaction rate. For this purpose, the rate constant is measured for two. The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as the pre.
from brunofuga.adv.br
Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. It is also known as the reaction rate. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress. The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as the pre. For this purpose, the rate constant is measured for two. If the rate constant doubles, for example,. The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases.
Arrhenius Constant Authorized Site brunofuga.adv.br
Rate Constant Arrhenius Definition The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress. Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. For this purpose, the rate constant is measured for two. The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress. The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as the pre. If the rate constant doubles, for example,. It is also known as the reaction rate. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate.
From www.researchgate.net
Arrhenius plots of the reaction rate constants for Reactions [1 Rate Constant Arrhenius Definition If the rate constant doubles, for example,. The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress. For this purpose, the rate constant is measured for two. Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. The. Rate Constant Arrhenius Definition.
From telegra.ph
Rate constant k units arrhenius equation for activation Telegraph Rate Constant Arrhenius Definition Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. For this purpose, the rate constant is measured for two. It is also known as the reaction rate. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. If the. Rate Constant Arrhenius Definition.
From www.youtube.com
Find the rate constant using the Arrhenius Equation YouTube Rate Constant Arrhenius Definition The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The arrhenius equation is an expression that provides a relationship between the rate constant. Rate Constant Arrhenius Definition.
From www.researchgate.net
Reaction rate constants Arrhenius dependence Download Scientific Diagram Rate Constant Arrhenius Definition The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. If the rate constant doubles, for example,. The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter. Rate Constant Arrhenius Definition.
From www.youtube.com
Temperature dependence theory of rate constant Arrhenius Equation Rate Constant Arrhenius Definition Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute. Rate Constant Arrhenius Definition.
From www.youtube.com
Temperature and Arrhenius equation YouTube Rate Constant Arrhenius Definition If the rate constant doubles, for example,. The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The arrhenius equation. Rate Constant Arrhenius Definition.
From www.researchgate.net
Arrhenius plot for the proton dissociation rate constant from wtGFP Rate Constant Arrhenius Definition The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress. It is also known as the reaction rate. The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. The rate constant is. Rate Constant Arrhenius Definition.
From www.pearson.com
The following data shows the rate constant of a reaction measured Rate Constant Arrhenius Definition Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. For this purpose, the rate constant is measured for two. If the rate constant doubles, for example,. The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. The arrhenius equation is an. Rate Constant Arrhenius Definition.
From www.researchgate.net
Arrhenius diagram for the rate constants k 1 and k 2 (s À1 Download Rate Constant Arrhenius Definition The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as the pre. For this purpose, the rate constant is measured for two. The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation. Rate Constant Arrhenius Definition.
From www.researchgate.net
Arrhenius plot of the rate constants. Download Scientific Diagram Rate Constant Arrhenius Definition Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. It is also known as the reaction rate. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. For this purpose, the rate constant is measured for two. If the. Rate Constant Arrhenius Definition.
From www.numerade.com
SOLVED The Arrhenius equation shows the relationship between the rate Rate Constant Arrhenius Definition The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as the pre. If the rate constant doubles, for example,. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction. Rate Constant Arrhenius Definition.
From telegra.ph
Rate constant k units arrhenius equation for activation Telegraph Rate Constant Arrhenius Definition Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. It is also known as the reaction rate. For this purpose, the rate constant is measured for two. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. If the. Rate Constant Arrhenius Definition.
From general.chemistrysteps.com
Arrhenius Equation Chemistry Steps Rate Constant Arrhenius Definition The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as the pre. Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. If the rate constant doubles, for example,. For this purpose, the rate constant is. Rate Constant Arrhenius Definition.
From www.researchgate.net
The Arrhenius rate constant, ln k , as a function of the inverse Rate Constant Arrhenius Definition The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress. It is also known as the reaction rate. If the rate constant doubles, for example,. For this purpose, the rate constant is measured for two. Arrhenius equation, mathematical expression that describes the effect of. Rate Constant Arrhenius Definition.
From www.slideserve.com
PPT The Arrhenius Equation PowerPoint Presentation, free download Rate Constant Arrhenius Definition For this purpose, the rate constant is measured for two. Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The rate constant, denoted as 'k' in the arrhenius equation,. Rate Constant Arrhenius Definition.
From www.shutterstock.com
Arrhenius Equation Rate Constant Stock Vector (Royalty Free) 1952055694 Rate Constant Arrhenius Definition The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. It is also known as the reaction rate. Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. For this purpose, the rate constant is measured for two. If the. Rate Constant Arrhenius Definition.
From www.researchgate.net
(a) Arrhenius plot of rate constant against frying temperatures at Rate Constant Arrhenius Definition If the rate constant doubles, for example,. The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as the pre. The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. The. Rate Constant Arrhenius Definition.
From www.dreamstime.com
Arrhenius Equation Physical Chemistry Science Vector Infographic Stock Rate Constant Arrhenius Definition For this purpose, the rate constant is measured for two. It is also known as the reaction rate. The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity. Rate Constant Arrhenius Definition.
From www.researchgate.net
4 Arrhenius expressions of bimolecular NCN reaction rate constants Rate Constant Arrhenius Definition If the rate constant doubles, for example,. It is also known as the reaction rate. The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. For this purpose, the rate constant is measured for two. The arrhenius equation is an expression that provides a relationship between the rate. Rate Constant Arrhenius Definition.
From www.researchgate.net
Overview of rate constants, represented in an Arrhenius plot, and the Rate Constant Arrhenius Definition The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress. Arrhenius equation, mathematical expression that describes the effect of temperature on the. Rate Constant Arrhenius Definition.
From www.slideserve.com
PPT The Arrhenius Equation PowerPoint Presentation, free download Rate Constant Arrhenius Definition If the rate constant doubles, for example,. The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. It is also known as the reaction rate. The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the. Rate Constant Arrhenius Definition.
From www.researchgate.net
Arrhenius diagram for the rate constants k 1 and k 2 (s À1 Download Rate Constant Arrhenius Definition The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as the pre. The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. For this purpose, the rate constant is measured. Rate Constant Arrhenius Definition.
From www.slideserve.com
PPT The Arrhenius Equation PowerPoint Presentation, free download Rate Constant Arrhenius Definition The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. If the rate constant doubles, for example,. The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. For this purpose, the rate constant is. Rate Constant Arrhenius Definition.
From www.chemistrylearner.com
Arrhenius Equation (Plot) Definition, Form, Variables, and Constants Rate Constant Arrhenius Definition The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress. Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. If the rate constant doubles, for example,. It is also known as the reaction rate. The arrhenius equation. Rate Constant Arrhenius Definition.
From www.researchgate.net
Arrhenius plots of the reaction rate constants k 1 and k 2 of reactions Rate Constant Arrhenius Definition The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress. Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. For this purpose, the rate constant is measured for two. The rate constant is a proportionality factor in. Rate Constant Arrhenius Definition.
From brunofuga.adv.br
Arrhenius Constant Authorized Site brunofuga.adv.br Rate Constant Arrhenius Definition The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as the pre. Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. The rate constant is a proportionality factor in the rate law of chemical kinetics. Rate Constant Arrhenius Definition.
From telegra.ph
Activation energy equation rate constant Telegraph Rate Constant Arrhenius Definition The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. For this purpose, the rate constant is measured for two. The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress.. Rate Constant Arrhenius Definition.
From www.researchgate.net
Arrhenius plot of the calculated rate constants for the NF 3 + H Rate Constant Arrhenius Definition It is also known as the reaction rate. Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The rate constant, denoted as 'k' in the arrhenius equation, serves as. Rate Constant Arrhenius Definition.
From rumble.com
Arrhenius Equation Activation Energy and Rate Constant K Explained Rate Constant Arrhenius Definition It is also known as the reaction rate. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as. Rate Constant Arrhenius Definition.
From www.researchgate.net
Arrhenius plots for the rate constants of the model. Download Rate Constant Arrhenius Definition Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. For this purpose, the rate constant is measured for two. If the rate constant doubles, for example,. The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known. Rate Constant Arrhenius Definition.
From www.researchgate.net
Arrhenius plot of agglomeration rate constant. Download Scientific Rate Constant Arrhenius Definition For this purpose, the rate constant is measured for two. If the rate constant doubles, for example,. It is also known as the reaction rate. The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as the pre. Arrhenius equation, mathematical expression that. Rate Constant Arrhenius Definition.
From www.researchgate.net
Arrhenius plot of the apparent rate constant. Download Scientific Diagram Rate Constant Arrhenius Definition Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. For this purpose, the rate constant is measured for two. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. The exponential term in the arrhenius equation implies that the. Rate Constant Arrhenius Definition.
From atwocab.blogspot.com
Arrhenius equation Increasing temperature increases the rate reaction Rate Constant Arrhenius Definition The exponential term in the arrhenius equation implies that the rate constant of a reaction increases exponentially when the activation energy decreases. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. If the rate constant doubles, for example,. For this purpose, the rate constant is. Rate Constant Arrhenius Definition.
From www.slideserve.com
PPT Reactions Rates and Temperature PowerPoint Presentation, free Rate Constant Arrhenius Definition The rate constant, denoted as 'k' in the arrhenius equation, serves as a pivotal parameter that encapsulates the intrinsic propensity of a chemical reaction to progress. The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as the pre. It is also known. Rate Constant Arrhenius Definition.
From www.researchgate.net
Arrhenius plots of rate constants derived from Figure 1. The linear fit Rate Constant Arrhenius Definition The arrhenius equation is an expression that provides a relationship between the rate constant (of a chemical reaction), the absolute temperature, and the a factor (also known as the pre. Arrhenius equation, mathematical expression that describes the effect of temperature on the velocity of a chemical. The rate constant is a proportionality factor in the rate law of chemical kinetics. Rate Constant Arrhenius Definition.