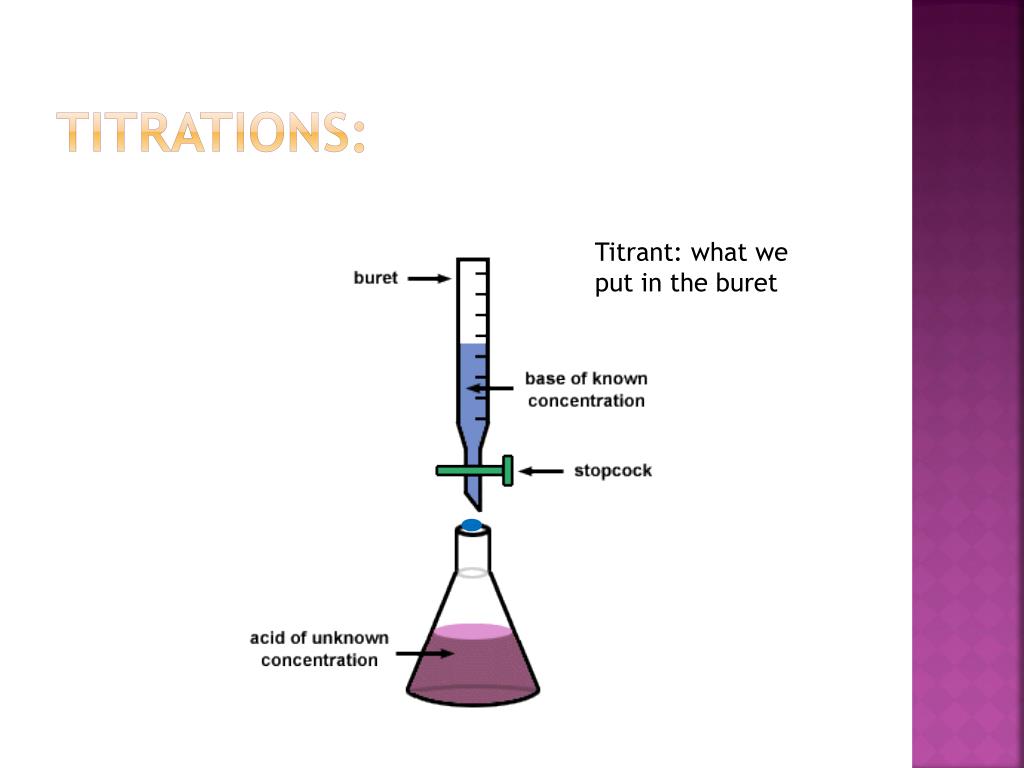
Titration Background Information . A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. It is based on the neutralization reaction , where.
from www.slideserve.com
Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. It is based on the neutralization reaction , where.
PPT Neutralization and Titration PowerPoint Presentation, free
Titration Background Information It is based on the neutralization reaction , where. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. It is based on the neutralization reaction , where. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
From studylib.net
Background Information (Titration) Lab III (iv) Titration is used in Titration Background Information It is based on the neutralization reaction , where. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a. Titration Background Information.
From dokumen.tips
(PDF) POTENTIOMETRIC TITRATIONS & SOLUBILITY...Revised 3/27/14 ! 5 Titration Background Information Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. Titration Background Information.
From www.aiophotoz.com
What Is A Titration Images and Photos finder Titration Background Information A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one solution of a known concentration. Titration Background Information.
From www.studocu.com
Titration Lab Organic Chemistry 1 Background This experiment focuses Titration Background Information Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution. Titration Background Information.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Background Information Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose. Titration Background Information.
From www.chegg.com
Solved Background Information Back Titrations*A back Titration Background Information Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a laboratory technique used to determine the. Titration Background Information.
From www.scribd.com
EDTA Titration Background PDF Titration Chemistry Titration Background Information A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant,. Titration Background Information.
From www.studocu.com
Lab report titration abstract Abstract Background information on Titration Background Information Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Titration Background Information.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Background Information Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Titration Background Information.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Background Information Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. It is based on the neutralization reaction , where. Titration is the slow addition of one. Titration Background Information.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources Titration Background Information Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose. Titration Background Information.
From www.slideserve.com
PPT Neutralization and Titration PowerPoint Presentation, free Titration Background Information Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. It is based on the neutralization reaction , where.. Titration Background Information.
From www.scribd.com
Titration PDF Titration Chemistry Titration Background Information Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. It is based on the neutralization reaction , where. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical. Titration Background Information.
From www.chegg.com
Solved Background Information Discuss what titrations are Titration Background Information It is based on the neutralization reaction , where. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown.. Titration Background Information.
From www.youtube.com
Titration background information YouTube Titration Background Information Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It is based on the neutralization reaction , where. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is the process of determining. Titration Background Information.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration Background Information A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration Background Information.
From fr.dreamstime.com
Technique De Titration Dans Le Laboratoire Photo stock Image du glace Titration Background Information Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose. Titration Background Information.
From www.compoundchem.com
Compound Interest Chemistry Techniques Titration Titration Background Information A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose. Titration Background Information.
From www.vectorstock.com
Design of chemical titration equipment Royalty Free Vector Titration Background Information A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titration. Titration Background Information.
From www.freepik.com
Premium AI Image A titration setup with a burette and a conical flask Titration Background Information Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. It is based on the neutralization reaction , where. Titration is a. Titration Background Information.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Background Information A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to. Titration Background Information.
From www.alamy.com
Acidbased titration equipment in chemistry laboratory vector Stock Titration Background Information Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely. Titration Background Information.
From www.scienceequip.com.au
Everything You Need To Know About Back Titration Science Equip Titration Background Information Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titration involves. Titration Background Information.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Background Information A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It is based on the neutralization reaction , where. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a. Titration Background Information.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Background Information A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a laboratory technique used to determine the concentration of an. Titration Background Information.
From vdocuments.mx
AcidBase Titrations Woodbridge Township School … Titrations Titration Background Information Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It is based on the neutralization reaction , where. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. A titration is a laboratory technique used to precisely measure. Titration Background Information.
From byjus.com
Why is titration used? Titration Background Information Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Titration Background Information.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Background Information Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a laboratory technique used to determine the concentration of an unknown chemical in. Titration Background Information.
From pdfslide.net
(PPT) Acid Base Titrations AP Chemistry Chapter 15. Titration Titration Background Information Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. It is. Titration Background Information.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Background Information Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is the process of determining the quantity of one substance by adding measured. Titration Background Information.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Titration Background Information It is based on the neutralization reaction , where. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the. Titration Background Information.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle Titration Background Information A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration Background Information.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID9336460 Titration Background Information Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. A titration is a laboratory technique used to precisely measure molar concentration. Titration Background Information.
From www.chegg.com
Solved Background Information Discuss what titrations are Titration Background Information A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It is based on the neutralization reaction , where. Titration involves the gradual addition of. Titration Background Information.
From slidetodoc.com
Analysis of Laundry Bleach An OxidationReduction Titration Tadas Titration Background Information It is based on the neutralization reaction , where. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of. Titration Background Information.