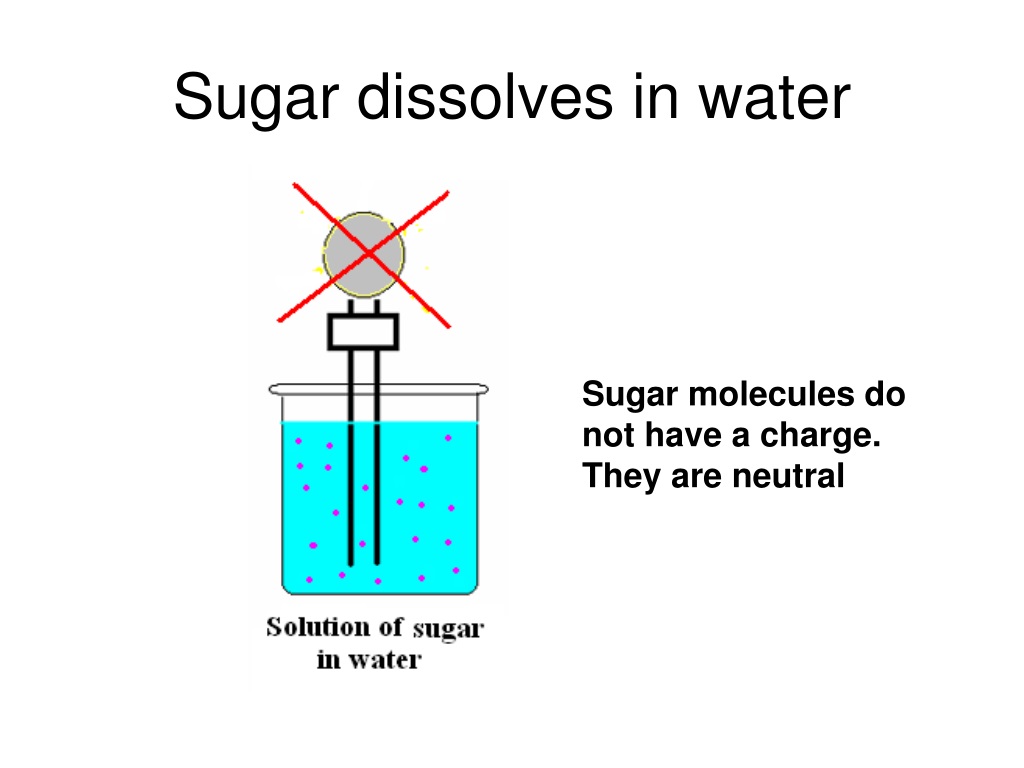
Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The . This process is called “dissolution.” Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. And, water is called the. In fact, nearly all the. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Water dissolves more substance than any other solvent. When these compounds dissolve in water, they do not. Once the sugar evenly disperses throughout the water, it becomes a solution. Water typically dissolves many ionic compounds and polar molecules. Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Which phrase best defines a covalent bond? Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Electrons shared between two atoms.
from www.slideserve.com
Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in. Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. Which phrase best defines a covalent bond? Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. When these compounds dissolve in water, they do not. In fact, nearly all the. Electrons shared between two atoms.
PPT Types of Solutes PowerPoint Presentation, free download ID9371021
Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water typically dissolves many ionic compounds and polar molecules. Water dissolves more substance than any other solvent. Water typically dissolves many ionic compounds and polar molecules. And, water is called the. Nonpolar molecules such as those found in grease or oil do not dissolve in. When these compounds dissolve in water, they do not. This process is called “dissolution.” Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. In fact, nearly all the. Once the sugar evenly disperses throughout the water, it becomes a solution. Electrons shared between two atoms. Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. Which phrase best defines a covalent bond? Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna.
From guidedehartfederalist.z21.web.core.windows.net
Kcl Dissolved In Water Diagram Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Once the sugar evenly disperses throughout the water, it becomes a solution. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. Nonpolar molecules such as those. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From sciencenotes.org
Why Is Water Called the Universal Solvent? Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The In fact, nearly all the. Once the sugar evenly disperses throughout the water, it becomes a solution. When these compounds dissolve in water, they do not. Water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in. Sugar and water, on the other hand, begin as a mixture. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3333021 Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water typically dissolves many ionic compounds and polar molecules. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Water dissolves more substance than any other solvent. Many molecular compounds, such a sugar or ethanol, are nonelectrolytes.. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Nonpolar molecules such as those found in grease or oil do not dissolve in. In fact, nearly all the. Which phrase best defines a covalent bond? Water dissolves more substance than any other solvent. Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. Water. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water dissolves more substance than any other solvent. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. This process is called “dissolution.” Which phrase best defines a covalent bond? But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Electrons shared between two atoms. Water is capable of dissolving a variety of different. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.slideserve.com
PPT Types of Solutes PowerPoint Presentation, free download ID9371021 Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Water typically dissolves many ionic compounds and polar molecules. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Nonpolar molecules such as those found. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From brentongokemckay.blogspot.com
What Happens When an Ionic Compound Dissolves in Water Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. This process is called “dissolution.” Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. When these compounds dissolve in water, they do not. Water is capable of dissolving a variety of different substances,. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The This process is called “dissolution.” Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Once the sugar evenly disperses throughout the water, it becomes a solution. Which phrase best defines a covalent bond? Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. Nonpolar molecules. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From wiringdiagrambig.z19.web.core.windows.net
Diagram Of Sugar Dissolving In Water Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The In fact, nearly all the. When these compounds dissolve in water, they do not. Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. Electrons shared between two atoms. Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. Water dissolves more substance than any other solvent. And,. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.youtube.com
1A 4.5 Types of Aqueous Solutions & Solubility YouTube Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. When these compounds dissolve in water, they do not. Electrons shared between two atoms. And, water is called the. Once the sugar evenly. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From slideplayer.com
Water and Life Processes ppt download Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Once the sugar evenly disperses throughout the water, it becomes a solution. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. This process is called “dissolution.” Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. Which phrase best defines a covalent bond? Water typically dissolves many ionic compounds and polar molecules.. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The When these compounds dissolve in water, they do not. In fact, nearly all the. And, water is called the. Water typically dissolves many ionic compounds and polar molecules. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Which phrase best defines a covalent bond? Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.slideserve.com
PPT Ionic vs. Molecular Compounds PowerPoint Presentation ID3074480 Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water typically dissolves many ionic compounds and polar molecules. Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. Water is capable of dissolving a variety of different substances, which is why it. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.slideserve.com
PPT Formation of a precipitate PowerPoint Presentation ID2860260 Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Which phrase best defines a covalent bond? Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Water typically dissolves many ionic compounds and polar molecules. Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. Nonpolar molecules such as those found in grease or oil. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From slideplayer.com
Water and Life Processes ppt download Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The This process is called “dissolution.” Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. When these compounds dissolve in water, they. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From study.com
Compound Solubility in Water Overview & Examples Lesson Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. Water dissolves polar molecules, including salts, sugars,. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.sciencelearn.org.nz
Sugar dissolving in water — Science Learning Hub Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The When these compounds dissolve in water, they do not. Nonpolar molecules such as those found in grease or oil do not dissolve in. Once the sugar evenly disperses throughout the water, it becomes a solution. Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Water. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Nonpolar molecules such as those found in grease or oil do not dissolve in. Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. In fact, nearly all the. Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. Water is often called the universal solvent because of its ability to dissolve so many chemicals. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From exozucqop.blob.core.windows.net
Water Can Dissolve Many Substances Due To Its Polarity And Hydrogen Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. And, water is called the. Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. Electrons shared between two atoms. This process is called “dissolution.” But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. When these compounds dissolve. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.slideserve.com
PPT Section 2 How Substances Dissolve PowerPoint Presentation, free Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water typically dissolves many ionic compounds and polar molecules. Which phrase best defines a covalent bond? Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. In fact, nearly. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From slideplayer.com
Water is the medium of life ppt download Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Which phrase best defines a covalent bond? Nonpolar molecules such as those found in grease or oil do not dissolve in. Electrons shared between two atoms. Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. Water dissolves more substance than any other solvent. Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. This. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation ID5875746 Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Water dissolves more substance than any other solvent. And, water is called the. Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. When these compounds dissolve. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From slideplayer.com
Water and Life Processes ppt download Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The When these compounds dissolve in water, they do not. Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. In fact, nearly all the. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Once the sugar evenly disperses. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water dissolves more substance than any other solvent. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. Sugar. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From engineerfix.com
Can Gases Dissolve In Water? (All You Need To Know) Engineer Fix Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water typically dissolves many ionic compounds and polar molecules. In fact, nearly all the. Nonpolar molecules such as those found in grease or oil do not dissolve in. Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. But, it isn’t a universal solvent because it can’t dissolve. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From slideplayer.com
Properties of Water. ppt download Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The And, water is called the. When these compounds dissolve in water, they do not. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Many molecular compounds, such a. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.slideserve.com
PPT Chemical and Physical Features of Seawater and the World Ocean Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water typically dissolves many ionic compounds and polar molecules. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. This process is called “dissolution.” Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. Electrons shared between two atoms. Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. In fact, nearly all the.. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Electrons shared between two atoms. And, water is called the. Water typically dissolves many ionic compounds and polar molecules. Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. Many molecular compounds, such. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From slideplayer.com
Properties of Water. ppt download Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The When these compounds dissolve in water, they do not. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Electrons shared between two atoms. Once the sugar evenly disperses throughout the water, it becomes a solution. This process is. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.slideserve.com
PPT Natural Approach to Chemistry Chapter 9 Water & Solutions Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water dissolves more substance than any other solvent. Water typically dissolves many ionic compounds and polar molecules. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Water is capable of dissolving a variety of different substances, which is why it is such a good solvent. Nonpolar molecules such as those found in grease or oil do not. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From courses.lumenlearning.com
The Dissolution Process Chemistry Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Electrons shared between two atoms. Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. In fact, nearly all the. This process is called “dissolution.” Water dissolves polar molecules, including salts, sugars, many gases, proteins, simple alcohols, and dna. And, water is called the. Nonpolar molecules such as those found in grease or oil do. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.slideserve.com
PPT Section 2 How Substances Dissolve PowerPoint Presentation, free Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Water typically dissolves many ionic compounds and polar molecules. But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. And, water is called the. This process is called “dissolution.” Water dissolves more substance than any other solvent. In fact, nearly all the. Many. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3188660 Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Many molecular compounds, such a sugar or ethanol, are nonelectrolytes. Once the sugar evenly disperses throughout the water, it becomes a solution. Sugar and water, on the other hand, begin as a mixture until the sugar dissolves. Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. Nonpolar. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The Which phrase best defines a covalent bond? Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. Water typically dissolves many ionic compounds and polar molecules. This process is called “dissolution.” But, it isn’t a universal solvent because it can’t dissolve hydrophobic or. Nonpolar molecules such as those. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.
From www.gbu-presnenskij.ru
Ions In Aqueous Solution Infographic Diagram Showing, 44 OFF Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The In fact, nearly all the. Water is often called the universal solvent because of its ability to dissolve so many chemicals to form homogeneous mixtures called solutions. When these compounds dissolve in water, they do not. Once the sugar evenly disperses throughout the water, it becomes a solution. Water is capable of dissolving a variety of different substances, which is. Water Can Dissolve Many Substances Like Ions Gases And Sugar. Water Is Considered The.