Reaction Between Hcl And Naoh . Hcl + naoh = nacl + h₂o + q. Consider the reaction between hydrochloric acid and sodium hydroxide; Hcl + naoh → nacl + h 2 o. Acid + base → salt and water. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. The general form of the reaction is an acid and a base react and form a salt and water: During the chemical reaction, the atoms rearrange to form the products of sodium. Alternatively, you can write the reaction as an ionic. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat.
from studylib.net
The general form of the reaction is an acid and a base react and form a salt and water: In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Hcl + naoh → nacl + h 2 o. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. Consider the reaction between hydrochloric acid and sodium hydroxide; Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. During the chemical reaction, the atoms rearrange to form the products of sodium. Hcl + naoh = nacl + h₂o + q. Acid + base → salt and water.
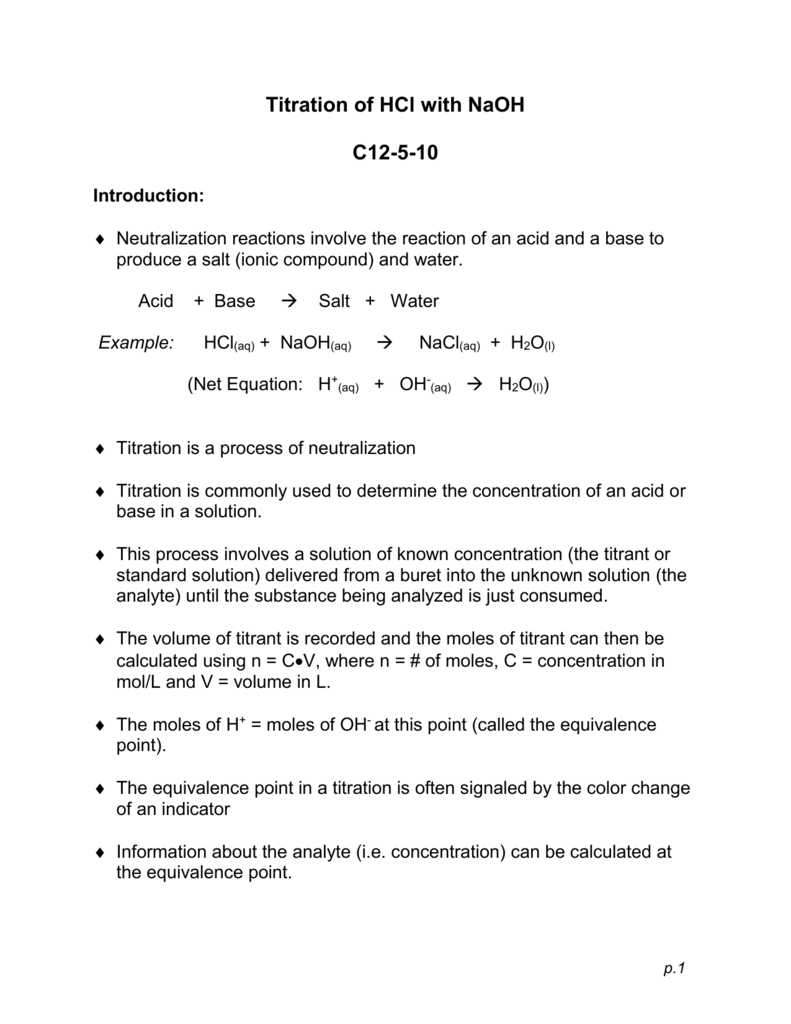
Titration of HCl with NaOH
Reaction Between Hcl And Naoh Hcl + naoh → nacl + h 2 o. Alternatively, you can write the reaction as an ionic. The general form of the reaction is an acid and a base react and form a salt and water: A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. During the chemical reaction, the atoms rearrange to form the products of sodium. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Acid + base → salt and water. Hcl + naoh → nacl + h 2 o. Hcl + naoh = nacl + h₂o + q. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Consider the reaction between hydrochloric acid and sodium hydroxide; Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase.
From www.slideshare.net
9 Aqueous Solutions Reaction Between Hcl And Naoh Acid + base → salt and water. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. Consider the reaction between hydrochloric acid and sodium hydroxide; In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Hcl + naoh = nacl. Reaction Between Hcl And Naoh.
From www.numerade.com
SOLVED HCl + NaOH —> NaCl + H2O What is the main classification of Reaction Between Hcl And Naoh In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). During the chemical reaction, the atoms rearrange to form the products of sodium. Hcl + naoh = nacl + h₂o + q. The general form of the reaction is an acid and a base react and form a salt and water:. Reaction Between Hcl And Naoh.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Reaction Between Hcl And Naoh Hcl + naoh = nacl + h₂o + q. Acid + base → salt and water. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). A strong acid and a strong base, such as hcl(aq) and. Reaction Between Hcl And Naoh.
From www.vrogue.co
Sodium Metal Reacts With Hydrochloric Acid vrogue.co Reaction Between Hcl And Naoh Hcl + naoh = nacl + h₂o + q. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. Alternatively, you can write the reaction as an ionic. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. The general form of the reaction is an. Reaction Between Hcl And Naoh.
From stock.adobe.com
Acid and base reactions. Neutralization reaction. HCl and NaOH reaction Reaction Between Hcl And Naoh A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. Hcl + naoh → nacl + h 2 o. In general, a neutralization reaction. Reaction Between Hcl And Naoh.
From www.chegg.com
Solved 2. The reaction between HCl and NaOH is expected to Reaction Between Hcl And Naoh Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Acid + base → salt and water. Consider the reaction between hydrochloric acid and sodium hydroxide; Hcl + naoh → nacl + h 2 o. The general form of the reaction is an acid and a base react and form a salt and water: Hcl +. Reaction Between Hcl And Naoh.
From eduvark.com
Reaction of HCL and NAOH' 2022 2023 EduVark Reaction Between Hcl And Naoh Consider the reaction between hydrochloric acid and sodium hydroxide; The general form of the reaction is an acid and a base react and form a salt and water: Acid + base → salt and water. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are. Reaction Between Hcl And Naoh.
From www.chegg.com
Solved A reaction between HCl and NaOH is being studied in a Reaction Between Hcl And Naoh Consider the reaction between hydrochloric acid and sodium hydroxide; Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. The general form of the reaction is an acid and a base react and form a salt and water: During the chemical reaction, the atoms rearrange to form the products of sodium. In general, a neutralization reaction. Reaction Between Hcl And Naoh.
From www.youtube.com
How to Balance NaOH + HCl = NaCl + H2O (Sodium Hydroxide Plus Reaction Between Hcl And Naoh Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. The general form of the reaction is an acid and a base react and form a salt and water: In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Consider the. Reaction Between Hcl And Naoh.
From www.chegg.com
Solved The neutralization reaction between HCl and NaOH is Reaction Between Hcl And Naoh A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Consider the reaction between hydrochloric acid and sodium hydroxide; In general, a neutralization reaction is a type of double replacement. Reaction Between Hcl And Naoh.
From www.youtube.com
Acid/Base Neutralization Reaction for NaOH + HCl (Sodium hydroxide Reaction Between Hcl And Naoh Consider the reaction between hydrochloric acid and sodium hydroxide; A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. Alternatively, you can write the reaction as an ionic. Acid + base → salt and water. Hcl + naoh = nacl + h₂o + q.. Reaction Between Hcl And Naoh.
From www.youtube.com
Heat of neutralisation for the reaction `NaOH+HCl to NaCl+H_(2)O` is Reaction Between Hcl And Naoh A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. Consider the reaction between hydrochloric acid and sodium hydroxide; Hcl + naoh = nacl + h₂o + q. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not. Reaction Between Hcl And Naoh.
From googglet.com
Neutralization reaction between hcl and naoh Reaction Between Hcl And Naoh Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Acid + base → salt and water. During the chemical reaction, the atoms rearrange to form the products of sodium. Alternatively, you can write the reaction as. Reaction Between Hcl And Naoh.
From eduvark.com
Reaction of HCL and NAOH' 2022 2023 EduVark Reaction Between Hcl And Naoh Acid + base → salt and water. Alternatively, you can write the reaction as an ionic. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Consider the reaction between hydrochloric acid and sodium hydroxide; Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. In. Reaction Between Hcl And Naoh.
From www.chegg.com
Results/Observations 1. Reaction Between HCl and NaOH Reaction Between Hcl And Naoh Hcl + naoh = nacl + h₂o + q. During the chemical reaction, the atoms rearrange to form the products of sodium. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. Reaction Between Hcl And Naoh.
From stock.adobe.com
Acidbase reaction. chemical reaction neutralization. HCl hydrochloric Reaction Between Hcl And Naoh Hcl + naoh = nacl + h₂o + q. The general form of the reaction is an acid and a base react and form a salt and water: Alternatively, you can write the reaction as an ionic. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). A strong acid and. Reaction Between Hcl And Naoh.
From www.youtube.com
Neutralization Reaction between HCl and NaOH Reaction Between HCl and Reaction Between Hcl And Naoh Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. The general form of the reaction is an acid and a base react and form a salt and water: A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. Hcl. Reaction Between Hcl And Naoh.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Reaction Between Hcl And Naoh In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Alternatively, you can write the reaction as an ionic. Consider the reaction between hydrochloric acid and sodium hydroxide; During the chemical reaction, the atoms rearrange to form the products of sodium. Hydrochloric acid and sodium hydroxide interact, resulting in salt and. Reaction Between Hcl And Naoh.
From www.markedbyteachers.com
Investigation of Energy changes in reaction between NaOH and HCL Reaction Between Hcl And Naoh Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. Hcl + naoh → nacl + h 2 o. The general form of the reaction is an acid and a base react and form a salt and water: Consider the reaction between hydrochloric acid and sodium hydroxide; Acid + base. Reaction Between Hcl And Naoh.
From www.numerade.com
SOLVED 21. In a neutralization reaction between NaOH and HCl, the Reaction Between Hcl And Naoh Hcl + naoh → nacl + h 2 o. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. A strong acid and a strong base, such as hcl(aq) and naoh(aq). Reaction Between Hcl And Naoh.
From eduvark.com
Reaction of HCL and NAOH' 2022 2023 EduVark Reaction Between Hcl And Naoh Consider the reaction between hydrochloric acid and sodium hydroxide; A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. During the chemical reaction, the atoms rearrange to form the products of sodium. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release. Reaction Between Hcl And Naoh.
From www.chegg.com
Solved Q13. The neutralization reaction between HCl and NaOH Reaction Between Hcl And Naoh Hcl + naoh = nacl + h₂o + q. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Consider the reaction between hydrochloric acid. Reaction Between Hcl And Naoh.
From byjus.com
The graph of pH during the titration of NaOH and HCl Reaction Between Hcl And Naoh Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Alternatively, you can write the reaction as an ionic. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). During the chemical reaction, the atoms rearrange to form the products of sodium. The general form of the. Reaction Between Hcl And Naoh.
From mungfali.com
HCl And NaOH Equation Reaction Between Hcl And Naoh Consider the reaction between hydrochloric acid and sodium hydroxide; During the chemical reaction, the atoms rearrange to form the products of sodium. Hcl + naoh → nacl + h 2 o. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. Hydrochloric acid and. Reaction Between Hcl And Naoh.
From stock.adobe.com
Neutralization Reaction Infographic Diagram with example of Reaction Between Hcl And Naoh Consider the reaction between hydrochloric acid and sodium hydroxide; In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Hcl + naoh → nacl + h 2 o. During the chemical reaction, the atoms rearrange to form. Reaction Between Hcl And Naoh.
From eduvark.com
Reaction of HCL and NAOH' 2022 2023 EduVark Reaction Between Hcl And Naoh Consider the reaction between hydrochloric acid and sodium hydroxide; In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Hcl + naoh → nacl + h 2 o. Hcl + naoh = nacl + h₂o + q. Alternatively, you can write the reaction as an ionic. Hydrochloric acid and sodium hydroxide. Reaction Between Hcl And Naoh.
From eduvark.com
Reaction of HCL and NAOH' 2022 2023 EduVark Reaction Between Hcl And Naoh Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. Hcl + naoh = nacl + h₂o + q. Alternatively, you can write the reaction as an ionic. During the chemical reaction, the atoms rearrange to form the products of sodium. A strong acid and a strong base, such as. Reaction Between Hcl And Naoh.
From www.youtube.com
acidbase reaction (HCl + NaOH) YouTube Reaction Between Hcl And Naoh Alternatively, you can write the reaction as an ionic. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. Hydrochloric acid and sodium hydroxide. Reaction Between Hcl And Naoh.
From meetingtarget11.gitlab.io
Ideal Naoh Hcl Balanced Equation What Is The Chemical For Photosynthesis Reaction Between Hcl And Naoh Alternatively, you can write the reaction as an ionic. The general form of the reaction is an acid and a base react and form a salt and water: Hcl + naoh → nacl + h 2 o. Acid + base → salt and water. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not. Reaction Between Hcl And Naoh.
From abigailkruwconner.blogspot.com
Enthalpy of Neutralization of Hcl and Naoh Value AbigailkruwConner Reaction Between Hcl And Naoh Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Hcl + naoh = nacl + h₂o + q. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. In general, a neutralization reaction is a type of double replacement reaction between an acid and a. Reaction Between Hcl And Naoh.
From www.numerade.com
SOLVED What is the molar ratio of acid to base for the neutralization Reaction Between Hcl And Naoh A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. The general form of the reaction is an acid and a base react and form a salt and water: Consider the reaction between hydrochloric acid and sodium hydroxide; Alternatively, you can write the reaction. Reaction Between Hcl And Naoh.
From www.numerade.com
SOLVED Write a balanced double displacement equation for the reaction Reaction Between Hcl And Naoh The general form of the reaction is an acid and a base react and form a salt and water: In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Hcl + naoh → nacl + h 2 o. A strong acid and a strong base, such as hcl(aq) and naoh(aq) will. Reaction Between Hcl And Naoh.
From studylib.net
Titration of HCl with NaOH Reaction Between Hcl And Naoh A strong acid and a strong base, such as hcl(aq) and naoh(aq) will react to form a neutral solution since the conjugate partners produced are of. Consider the reaction between hydrochloric acid and sodium hydroxide; Alternatively, you can write the reaction as an ionic. Hcl + naoh = nacl + h₂o + q. The general form of the reaction is. Reaction Between Hcl And Naoh.
From abigailkruwconner.blogspot.com
Enthalpy of Neutralization of Hcl and Naoh Value AbigailkruwConner Reaction Between Hcl And Naoh Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. Consider the reaction between hydrochloric acid and sodium hydroxide; Acid + base → salt and water. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. A strong acid and a strong base, such as hcl(aq). Reaction Between Hcl And Naoh.
From mungfali.com
HCl And NaOH Net Ionic Equation Reaction Between Hcl And Naoh Alternatively, you can write the reaction as an ionic. Hydrochloric acid and sodium hydroxide interact, resulting in salt and a release of heat. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base (alkali). Acid + base → salt and water. The general form of the reaction is an acid and a. Reaction Between Hcl And Naoh.