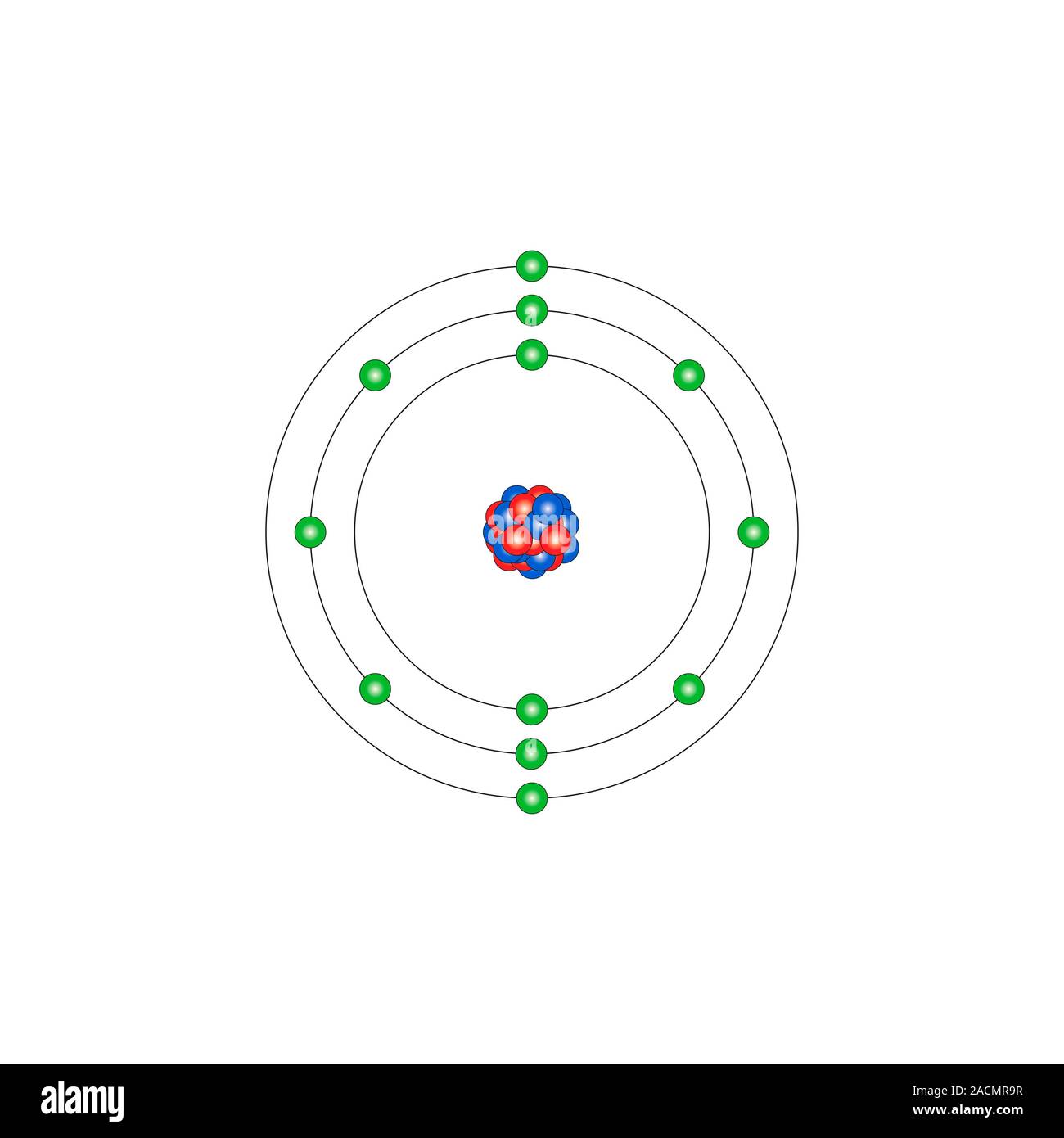
Magnesium Ion With Electrons . What is the total number of electrons in a m g2+ ion? Electron configuration of magnesium is [ne] 3s2. The easiest way to do this is to simply look at the periodic table, find the. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. Magnesium has two valence electrons in the 3s orbital. This would make it a. Possible oxidation states are +2. The ground state electron configuration of magnesium is important in understanding its. It is an essential ion for many. The symbol for the ion is mg 2 + ,. Magnesium occurs naturally only in combination with other. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons.
from www.animalia-life.club
The ground state electron configuration of magnesium is important in understanding its. This would make it a. It is an essential ion for many. Magnesium occurs naturally only in combination with other. Electron configuration of magnesium is [ne] 3s2. (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. What is the total number of electrons in a m g2+ ion? The symbol for the ion is mg 2 + ,. Magnesium has two valence electrons in the 3s orbital. Possible oxidation states are +2.
Magnesium Electron Configuration
Magnesium Ion With Electrons The easiest way to do this is to simply look at the periodic table, find the. Possible oxidation states are +2. It is an essential ion for many. The ground state electron configuration of magnesium is important in understanding its. What is the total number of electrons in a m g2+ ion? The symbol for the ion is mg 2 + ,. This would make it a. (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. Electron configuration of magnesium is [ne] 3s2. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. Magnesium occurs naturally only in combination with other. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. The easiest way to do this is to simply look at the periodic table, find the. Magnesium has two valence electrons in the 3s orbital.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Magnesium Ion With Electrons Possible oxidation states are +2. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. This would make it a. Magnesium has two valence electrons in the 3s orbital. The symbol for the ion is. Magnesium Ion With Electrons.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Magnesium Ion With Electrons Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The easiest way to do this is to simply look at the periodic table, find the. What is the total number of electrons in a m g2+ ion? (a) since mg 2 + is a cation, its name is the name. Magnesium Ion With Electrons.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Protons are represented Magnesium Ion With Electrons Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. The easiest way to do this is to simply look at the periodic table, find the. Magnesium occurs naturally only in combination with other. Magnesium has two valence electrons in the 3s orbital. Thus, a magnesium atom will form a cation with two fewer. Magnesium Ion With Electrons.
From www.dreamstime.com
Atom of Magnesium with Detailed Core and 12 Electrons on White with Atoms Stock Illustration Magnesium Ion With Electrons The easiest way to do this is to simply look at the periodic table, find the. It is an essential ion for many. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. (a) since mg 2 + is a cation, its name is the name of the element it comes. Magnesium Ion With Electrons.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Magnesium Ion With Electrons (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. This would. Magnesium Ion With Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion With Electrons Electron configuration of magnesium is [ne] 3s2. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. What is the total number of electrons in a m g2+ ion? It is an essential ion for. Magnesium Ion With Electrons.
From valenceelectrons.com
How Many Protons,Neutrons and Electrons Does Magnesium Have? Magnesium Ion With Electrons Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. It is an essential ion for many. This would make it a. Magnesium occurs naturally only in combination with other. Electron configuration of magnesium is [ne] 3s2. (a) since mg 2 + is a cation, its name is the name of. Magnesium Ion With Electrons.
From www.shutterstock.com
Diagram Representation Element Magnesium Neutrons Protons Stock Vector 328554326 Shutterstock Magnesium Ion With Electrons The ground state electron configuration of magnesium is important in understanding its. The easiest way to do this is to simply look at the periodic table, find the. (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. What is the total number of electrons in a. Magnesium Ion With Electrons.
From nursehub.com
Electron Shells NurseHub Magnesium Ion With Electrons What is the total number of electrons in a m g2+ ion? Electron configuration of magnesium is [ne] 3s2. The easiest way to do this is to simply look at the periodic table, find the. The ground state electron configuration of magnesium is important in understanding its. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom. Magnesium Ion With Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion With Electrons Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. The easiest way to do this is to simply look at the periodic table, find the. This would make it a. The symbol for the ion is mg 2 + ,. Magnesium has two valence electrons in the 3s orbital. Electron configuration of magnesium. Magnesium Ion With Electrons.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Ion With Electrons This would make it a. Possible oxidation states are +2. The symbol for the ion is mg 2 + ,. The easiest way to do this is to simply look at the periodic table, find the. Magnesium occurs naturally only in combination with other. Electron configuration of magnesium is [ne] 3s2. Magnesium has two valence electrons in the 3s orbital.. Magnesium Ion With Electrons.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Magnesium Ion With Electrons The symbol for the ion is mg 2 + ,. This would make it a. Possible oxidation states are +2. Electron configuration of magnesium is [ne] 3s2. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. The easiest way to do this is to simply look at the periodic table, find the. Thus,. Magnesium Ion With Electrons.
From www.slideserve.com
PPT Ionic Bonding L.O. PowerPoint Presentation, free download ID3763847 Magnesium Ion With Electrons Possible oxidation states are +2. The easiest way to do this is to simply look at the periodic table, find the. This would make it a. The symbol for the ion is mg 2 + ,. Electron configuration of magnesium is [ne] 3s2. It is an essential ion for many. Magnesium occurs naturally only in combination with other. Magnesium has. Magnesium Ion With Electrons.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Ion With Electrons Electron configuration of magnesium is [ne] 3s2. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. What is the total number of electrons in a m g2+ ion? The ground state electron configuration of magnesium is important in understanding its. Magnesium ion (mg2+) is a positively charged ion formed when. Magnesium Ion With Electrons.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Ion With Electrons Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. This would make it a. The easiest way to do this is to simply look at the periodic table, find the. Magnesium occurs naturally only in combination with other. What is the total number of electrons in a m g2+ ion?. Magnesium Ion With Electrons.
From ar.inspiredpencil.com
Magnesium Atom Structure Magnesium Ion With Electrons The ground state electron configuration of magnesium is important in understanding its. The symbol for the ion is mg 2 + ,. This would make it a. Magnesium has two valence electrons in the 3s orbital. (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. Electron. Magnesium Ion With Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Ion With Electrons This would make it a. Electron configuration of magnesium is [ne] 3s2. Magnesium has two valence electrons in the 3s orbital. It is an essential ion for many. (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. What is the total number of electrons in a. Magnesium Ion With Electrons.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Magnesium Ion With Electrons This would make it a. The ground state electron configuration of magnesium is important in understanding its. The easiest way to do this is to simply look at the periodic table, find the. Magnesium has two valence electrons in the 3s orbital. The symbol for the ion is mg 2 + ,. It is an essential ion for many. Magnesium. Magnesium Ion With Electrons.
From www.flexiprep.com
NCERT Class 9 Science Solutions Chapter 3 Atoms and Molecules Part 9 (For CBSE, ICSE, IAS, NET Magnesium Ion With Electrons Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. The symbol for the ion is mg 2 + ,. What is the total number of electrons in a m g2+ ion? The easiest way to do this is to simply look at the periodic table, find the. Electron configuration of magnesium is [ne]. Magnesium Ion With Electrons.
From www.istockphoto.com
Mg Magnesium Element Information Facts Properties Trends Uses And Comparison Periodic Table Of Magnesium Ion With Electrons Possible oxidation states are +2. Electron configuration of magnesium is [ne] 3s2. The symbol for the ion is mg 2 + ,. It is an essential ion for many. The ground state electron configuration of magnesium is important in understanding its. Magnesium occurs naturally only in combination with other. The easiest way to do this is to simply look at. Magnesium Ion With Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion With Electrons It is an essential ion for many. What is the total number of electrons in a m g2+ ion? Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The easiest way to do this is to simply look at the periodic table, find the. This would make it a. Magnesium. Magnesium Ion With Electrons.
From www.nagwa.com
Question Video Identifying the Energy Level Diagram That Represents the Electronic Magnesium Ion With Electrons This would make it a. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. It is an essential ion for many. Electron configuration of magnesium is [ne] 3s2. The easiest way to do this is to simply look at the periodic table, find the. Possible oxidation states are +2. (a) since mg 2. Magnesium Ion With Electrons.
From ar.inspiredpencil.com
Magnesium Atom Structure Magnesium Ion With Electrons The symbol for the ion is mg 2 + ,. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. Possible oxidation states are +2. What is the total number of electrons in a m g2+ ion? The easiest way to do this is to simply look at the periodic table,. Magnesium Ion With Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion With Electrons It is an essential ion for many. The ground state electron configuration of magnesium is important in understanding its. (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. The easiest way to do this is to simply look at the periodic table, find the. Thus, a. Magnesium Ion With Electrons.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Magnesium Ion With Electrons Possible oxidation states are +2. The ground state electron configuration of magnesium is important in understanding its. (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. What is the total number of electrons in a m g2+ ion? It is an essential ion for many. Electron. Magnesium Ion With Electrons.
From www.schoolmykids.com
Magnesium (Mg) Element Information, Facts, Properties, Uses Periodic Table of the Elements Magnesium Ion With Electrons (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. The easiest way to do this is to simply look at the periodic table, find the. It is an essential ion for many. The ground state electron configuration of magnesium is important in understanding its. The symbol. Magnesium Ion With Electrons.
From ar.inspiredpencil.com
Magnesium Electron Configuration Magnesium Ion With Electrons Magnesium has two valence electrons in the 3s orbital. Possible oxidation states are +2. The ground state electron configuration of magnesium is important in understanding its. It is an essential ion for many. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg 2. Magnesium Ion With Electrons.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion With Electrons The symbol for the ion is mg 2 + ,. What is the total number of electrons in a m g2+ ion? Possible oxidation states are +2. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. This would make it a. Magnesium occurs naturally only in combination with other. Magnesium has two valence. Magnesium Ion With Electrons.
From commons.wikimedia.org
FileElectron shell 012 Magnesium.svg Wikimedia Commons Magnesium Ion With Electrons Magnesium has two valence electrons in the 3s orbital. The ground state electron configuration of magnesium is important in understanding its. It is an essential ion for many. Electron configuration of magnesium is [ne] 3s2. The easiest way to do this is to simply look at the periodic table, find the. The symbol for the ion is mg 2 +. Magnesium Ion With Electrons.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Ion With Electrons This would make it a. It is an essential ion for many. The symbol for the ion is mg 2 + ,. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. Magnesium has two valence electrons in the 3s orbital. The easiest way to do this is to simply look at the periodic. Magnesium Ion With Electrons.
From mungfali.com
Magnesium Ion Electron Configuration Magnesium Ion With Electrons (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. Magnesium occurs naturally only in combination with other. It is an essential ion for many. Possible oxidation states are +2. The symbol for the ion is mg 2 + ,. The ground state electron configuration of magnesium. Magnesium Ion With Electrons.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Ion With Electrons (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. Magnesium occurs naturally only in combination with other. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. Thus, a magnesium atom will form a cation with two fewer electrons than. Magnesium Ion With Electrons.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Magnesium Ion With Electrons Electron configuration of magnesium is [ne] 3s2. Possible oxidation states are +2. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. It is an essential ion for many. This would make it a. Magnesium has two valence electrons in the 3s orbital. Thus, a magnesium atom will form a cation with two fewer. Magnesium Ion With Electrons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion With Electrons Magnesium occurs naturally only in combination with other. Possible oxidation states are +2. (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. It is an essential ion for many. Magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. Thus,. Magnesium Ion With Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Ion With Electrons (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. The ground state electron configuration of magnesium is important in understanding its. What is the total number of electrons in a m g2+ ion? Possible oxidation states are +2. The symbol for the ion is mg 2. Magnesium Ion With Electrons.