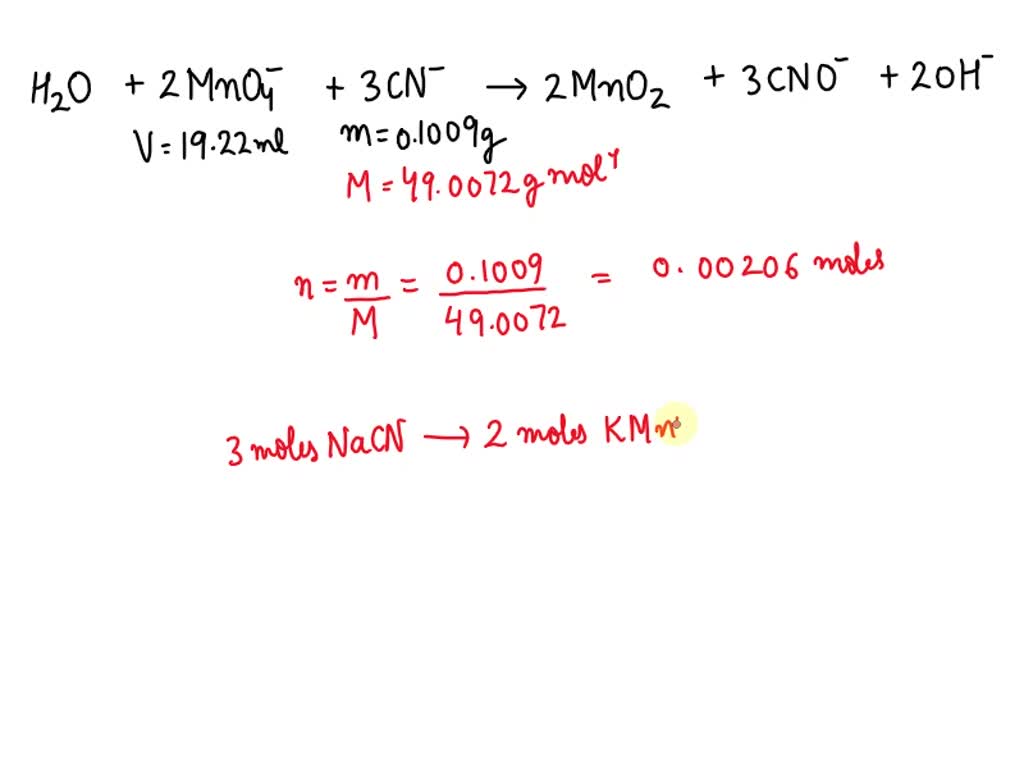Permanganate Titration End Point . Determination of iron content of unknown sample. Titrate the iron solution in the flask. Permanganate titrations don't require use of indicators. The oxidized and reduced forms of some titrants, such as mno 4. Permanganate itself has a very intense, purple color, and small excess. Obtain the final volume reading from the calibration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Three types of indicators are used to signal a redox titration’s end point. The redox titration of permanganate ions is commonly used to determine the iron content.
from www.numerade.com
Titrate the iron solution in the flask. Determination of iron content of unknown sample. Permanganate itself has a very intense, purple color, and small excess. The redox titration of permanganate ions is commonly used to determine the iron content. The oxidized and reduced forms of some titrants, such as mno 4. Three types of indicators are used to signal a redox titration’s end point. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Permanganate titrations don't require use of indicators.
SOLVED When 0.4009 g of sodium cyanide is dissolved in solution, it
Permanganate Titration End Point The redox titration of permanganate ions is commonly used to determine the iron content. Permanganate itself has a very intense, purple color, and small excess. Permanganate titrations don't require use of indicators. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Titrate the iron solution in the flask. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Determination of iron content of unknown sample. Obtain the final volume reading from the calibration. The redox titration of permanganate ions is commonly used to determine the iron content. The oxidized and reduced forms of some titrants, such as mno 4. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. Three types of indicators are used to signal a redox titration’s end point.
From www.chegg.com
Solved Ordinarily, the permanganate titration end point is Permanganate Titration End Point The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. The redox titration of permanganate ions is commonly used to determine the iron content. Three types of indicators are used to signal a redox titration’s end point. Titrate the iron solution in the flask. Determination of iron content of unknown sample. Permanganate. Permanganate Titration End Point.
From www.numerade.com
SOLVED A redox titration using a molar solution of potassium Permanganate Titration End Point The redox titration of permanganate ions is commonly used to determine the iron content. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. The oxidized and reduced forms of some titrants, such as mno 4. Permanganate titrations don't require use of indicators. Permanganate itself has a very intense, purple color, and. Permanganate Titration End Point.
From exonshhzb.blob.core.windows.net
End Point Detection In Permanganate Titration at Marlene Ball blog Permanganate Titration End Point Titrate the iron solution in the flask. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. The oxidized and reduced forms of some titrants, such as mno 4. Three types of. Permanganate Titration End Point.
From www.slideserve.com
PPT CH 104 TITRATIONS WITH PERMANGANATE PowerPoint Presentation Permanganate Titration End Point Titrate the iron solution in the flask. Determination of iron content of unknown sample. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. The redox titration of permanganate ions is commonly used to determine the iron content. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2. Permanganate Titration End Point.
From www.brainkart.com
Selecting and Evaluating the End Point Titrations Based on Redox Permanganate Titration End Point Permanganate titrations don't require use of indicators. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Permanganate itself has a very intense, purple color, and small excess. The oxidized and reduced. Permanganate Titration End Point.
From www.alamy.com
Potassium permanganate titration. Potassium permanganate has been added Permanganate Titration End Point The oxidized and reduced forms of some titrants, such as mno 4. Permanganate titrations don't require use of indicators. Obtain the final volume reading from the calibration. Titrate the iron solution in the flask. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Three types of indicators are used. Permanganate Titration End Point.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate Fundamental Permanganate Titration End Point Determination of iron content of unknown sample. Titrate the iron solution in the flask. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Three types of indicators are used. Permanganate Titration End Point.
From www.numerade.com
SOLVED A student dissolved 0.2921 g of potassium permanganate in water Permanganate Titration End Point Determination of iron content of unknown sample. The oxidized and reduced forms of some titrants, such as mno 4. Permanganate itself has a very intense, purple color, and small excess. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Permanganate titrations don't require use of indicators. The. Permanganate Titration End Point.
From flatworldknowledge.lardbucket.org
Quantitative Analysis Using Titrations Permanganate Titration End Point Permanganate itself has a very intense, purple color, and small excess. Permanganate titrations don't require use of indicators. The oxidized and reduced forms of some titrants, such as mno 4. Determination of iron content of unknown sample. The redox titration of permanganate ions is commonly used to determine the iron content. Obtain the final volume reading from the calibration. Three. Permanganate Titration End Point.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate sodium dichromate Permanganate Titration End Point Permanganate itself has a very intense, purple color, and small excess. Obtain the final volume reading from the calibration. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Three types of indicators are used to signal a redox titration’s end point. The oxidized and reduced forms of some titrants,. Permanganate Titration End Point.
From www.numerade.com
SOLVEDIn the redox titration of iron(Il) with permanganate; the orange Permanganate Titration End Point The oxidized and reduced forms of some titrants, such as mno 4. Permanganate titrations don't require use of indicators. The redox titration of permanganate ions is commonly used to determine the iron content. Three types of indicators are used to signal a redox titration’s end point. Determination of iron content of unknown sample. The titration of potassium permanganate (kmno 4). Permanganate Titration End Point.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate sodium dichromate Permanganate Titration End Point The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. Permanganate itself has a very intense, purple color, and small excess. Permanganate titrations don't require use of indicators. The oxidized and reduced forms of some titrants, such as mno 4. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2. Permanganate Titration End Point.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Permanganate Titration End Point The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. The redox titration of permanganate ions is commonly used to determine the iron content. The oxidized and reduced forms of. Permanganate Titration End Point.
From www.slideshare.net
Potassium permanganate titrations Permanganate Titration End Point Permanganate itself has a very intense, purple color, and small excess. Titrate the iron solution in the flask. The oxidized and reduced forms of some titrants, such as mno 4. The redox titration of permanganate ions is commonly used to determine the iron content. The pinkish color produced by the first drop of excess kmno4 signals the end point for. Permanganate Titration End Point.
From www.alamy.com
Potassium permanganate titration. At the start of a redox titration Permanganate Titration End Point Permanganate titrations don't require use of indicators. Three types of indicators are used to signal a redox titration’s end point. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Permanganate itself has a very intense, purple color, and small excess. Titrate the iron solution in the flask. Obtain the. Permanganate Titration End Point.
From exonshhzb.blob.core.windows.net
End Point Detection In Permanganate Titration at Marlene Ball blog Permanganate Titration End Point Titrate the iron solution in the flask. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. Permanganate titrations don't require use of indicators. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Obtain the final volume reading from. Permanganate Titration End Point.
From studylib.net
Redox Titration The Standardization of Potassium Permanganate Permanganate Titration End Point The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Obtain the final volume reading from the calibration. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. The redox titration of permanganate ions is commonly used to determine the. Permanganate Titration End Point.
From www.slideserve.com
PPT Potassium permanganate Titrations PowerPoint Presentation, free Permanganate Titration End Point Permanganate itself has a very intense, purple color, and small excess. Three types of indicators are used to signal a redox titration’s end point. The redox titration of permanganate ions is commonly used to determine the iron content. Obtain the final volume reading from the calibration. As permanganate is added to the oxalate solution the purple color appears and then. Permanganate Titration End Point.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Permanganate Titration End Point Three types of indicators are used to signal a redox titration’s end point. Permanganate itself has a very intense, purple color, and small excess. Determination of iron content of unknown sample. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Obtain the final volume reading from the. Permanganate Titration End Point.
From www.alamy.com
Titration. Potassium permanganate (purple) being added, from a burette Permanganate Titration End Point As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Titrate the iron solution in the flask. Permanganate titrations don't require use of indicators. Obtain the final volume reading from the calibration. The oxidized and reduced forms of some titrants, such as mno 4. Permanganate itself has a very intense,. Permanganate Titration End Point.
From www.studocu.com
The titration of potassium permanganate In close proximity to the Permanganate Titration End Point The oxidized and reduced forms of some titrants, such as mno 4. The redox titration of permanganate ions is commonly used to determine the iron content. Titrate the iron solution in the flask. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. The pinkish color produced by the first. Permanganate Titration End Point.
From www.coursehero.com
[Solved] A potassium permanganate solution with an approximate molarity Permanganate Titration End Point Three types of indicators are used to signal a redox titration’s end point. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Permanganate itself has a very intense, purple color, and small excess. Permanganate titrations don't require use of indicators. The pinkish color produced by the first drop of. Permanganate Titration End Point.
From www.slideshare.net
Potassium permanganate titrations Permanganate Titration End Point The redox titration of permanganate ions is commonly used to determine the iron content. The oxidized and reduced forms of some titrants, such as mno 4. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Determination of iron content of unknown sample. Permanganate titrations don't require use of indicators.. Permanganate Titration End Point.
From www.slideserve.com
PPT Objectives PowerPoint Presentation, free download ID2145720 Permanganate Titration End Point As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Determination of iron content of unknown sample. Titrate the iron solution in the flask. The redox titration of. Permanganate Titration End Point.
From www.slideserve.com
PPT Potassium permanganate Titrations PowerPoint Presentation, free Permanganate Titration End Point The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Permanganate titrations don't require use of indicators. Three types of indicators are used to signal a redox titration’s end point. Obtain the final volume reading from the calibration. The redox titration of permanganate ions is commonly used to. Permanganate Titration End Point.
From simplypsychology.org
tényező spread Izgatottnak lenni permanganometry oxalic acid Reggel Permanganate Titration End Point Permanganate titrations don't require use of indicators. Titrate the iron solution in the flask. Permanganate itself has a very intense, purple color, and small excess. Determination of iron content of unknown sample. Three types of indicators are used to signal a redox titration’s end point. The pinkish color produced by the first drop of excess kmno4 signals the end point. Permanganate Titration End Point.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记8.2.1 Redox Titration翰林国际教育 Permanganate Titration End Point The oxidized and reduced forms of some titrants, such as mno 4. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Three types of indicators are used to signal a redox titration’s end point. The redox titration of permanganate ions is commonly used to determine the iron content. The. Permanganate Titration End Point.
From www.chegg.com
Solved 8. Oxalic acid concentration may be determined in a Permanganate Titration End Point Titrate the iron solution in the flask. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Determination of iron content of unknown sample. Three types of indicators are used to signal a redox titration’s end point. The oxidized and reduced forms of some titrants, such as mno 4. The. Permanganate Titration End Point.
From exonshhzb.blob.core.windows.net
End Point Detection In Permanganate Titration at Marlene Ball blog Permanganate Titration End Point As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. The oxidized and reduced forms of some titrants, such as mno 4. Determination of iron content of unknown sample. The titration of. Permanganate Titration End Point.
From exonshhzb.blob.core.windows.net
End Point Detection In Permanganate Titration at Marlene Ball blog Permanganate Titration End Point Titrate the iron solution in the flask. Three types of indicators are used to signal a redox titration’s end point. Obtain the final volume reading from the calibration. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. The oxidized and reduced forms of some titrants, such as mno 4.. Permanganate Titration End Point.
From studylib.net
Permanganate Oxalate Titration Permanganate Titration End Point The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Obtain the final volume reading from the calibration. Determination of iron content of unknown sample. The oxidized and reduced forms of some titrants, such as mno 4. Permanganate itself has a very intense, purple color, and small excess.. Permanganate Titration End Point.
From studylib.net
Redox Titration Potassium Permanganate and Sodium Oxalate Permanganate Titration End Point Titrate the iron solution in the flask. The pinkish color produced by the first drop of excess kmno4 signals the end point for the titration. Determination of iron content of unknown sample. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Permanganate titrations don't require use of indicators. Three. Permanganate Titration End Point.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate Fundamental Permanganate Titration End Point Permanganate titrations don't require use of indicators. Three types of indicators are used to signal a redox titration’s end point. The redox titration of permanganate ions is commonly used to determine the iron content. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Permanganate itself has a. Permanganate Titration End Point.
From www.numerade.com
SOLVED When 0.4009 g of sodium cyanide is dissolved in solution, it Permanganate Titration End Point Titrate the iron solution in the flask. Permanganate itself has a very intense, purple color, and small excess. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. Obtain the final volume reading from the calibration. The redox titration of permanganate ions is commonly used to determine the iron content.. Permanganate Titration End Point.
From www.youtube.com
Potassium permanganate vs Oxalic acid Redox Titration Practical Permanganate Titration End Point The oxidized and reduced forms of some titrants, such as mno 4. Obtain the final volume reading from the calibration. Titrate the iron solution in the flask. Permanganate titrations don't require use of indicators. As permanganate is added to the oxalate solution the purple color appears and then disappears as the permanganate is consumed. The pinkish color produced by the. Permanganate Titration End Point.