Standard Heat Formation Of Copper Oxide . \[\delta h_{reaction}^o = \sum {\delta. Copper(ii) oxide permanent link for this species. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Use this link for bookmarking this species for future reference. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. To find the δh reaction o, use the formula for the standard enthalpy change of formation:
from www.mdpi.com
Copper(ii) oxide permanent link for this species. \[\delta h_{reaction}^o = \sum {\delta. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. To find the δh reaction o, use the formula for the standard enthalpy change of formation: The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Use this link for bookmarking this species for future reference.
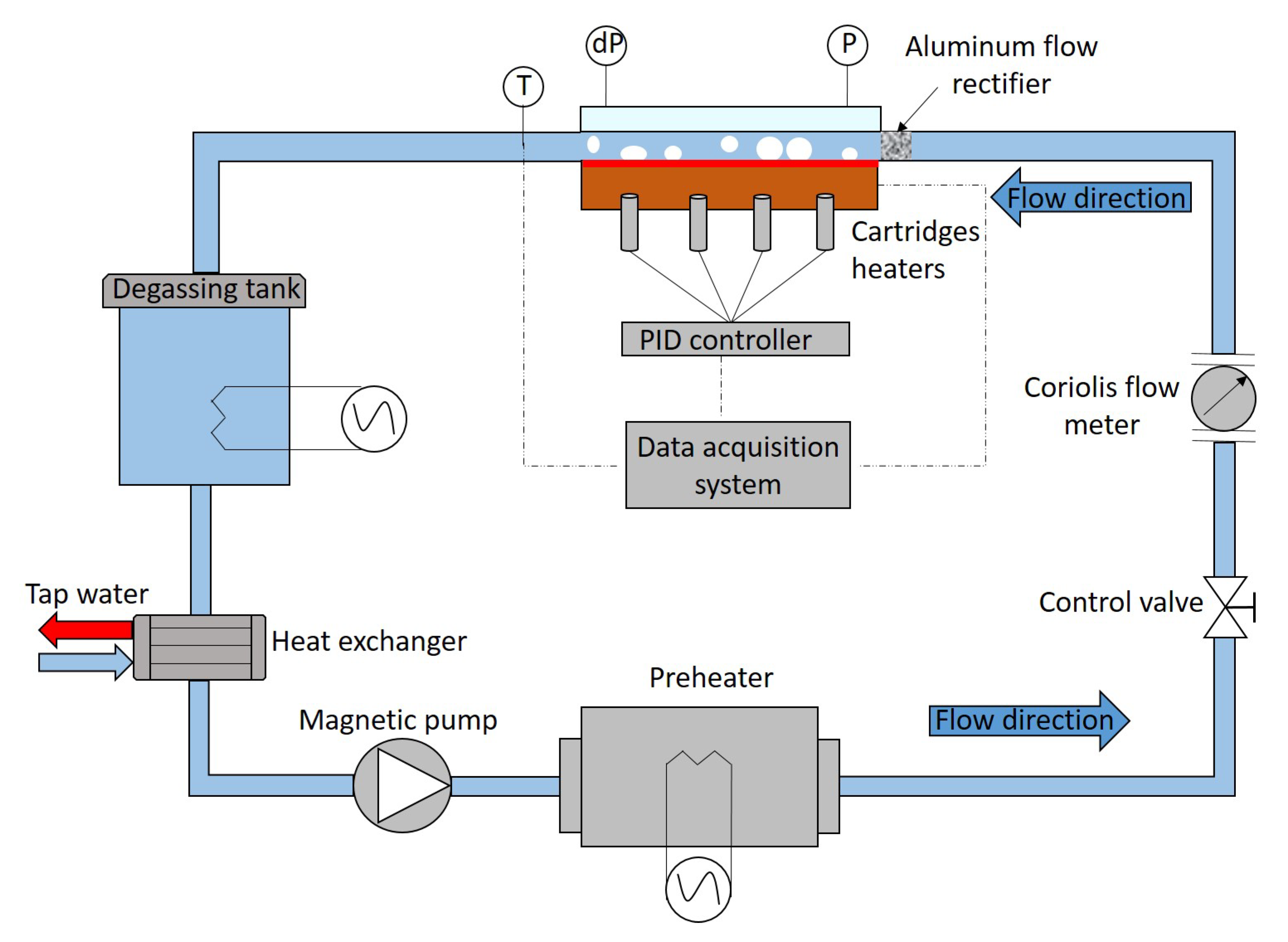
Applied Mechanics Free FullText Heat Transfer Deterioration by the
Standard Heat Formation Of Copper Oxide \[\delta h_{reaction}^o = \sum {\delta. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation of any element in its standard state is zero by definition. To find the δh reaction o, use the formula for the standard enthalpy change of formation: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Copper(ii) oxide permanent link for this species. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Use this link for bookmarking this species for future reference. \[\delta h_{reaction}^o = \sum {\delta. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including.
From www.fphoto.com
Fundamental Photographs The Art of Science Standard Heat Formation Of Copper Oxide \[\delta h_{reaction}^o = \sum {\delta. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard enthalpy change. Standard Heat Formation Of Copper Oxide.
From www.slideshare.net
Chapter 4 Standard Heat Formation Of Copper Oxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from. Standard Heat Formation Of Copper Oxide.
From www.researchgate.net
Standard free energy of formation of metal oxides as a function of Standard Heat Formation Of Copper Oxide For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. To find the δh reaction o, use the formula for the standard enthalpy change of formation: Use this link for bookmarking this species for. Standard Heat Formation Of Copper Oxide.
From www.researchgate.net
Graphical representation of biomediated synthesis of copper oxide Standard Heat Formation Of Copper Oxide Use this link for bookmarking this species for future reference. To find the δh reaction o, use the formula for the standard enthalpy change of formation: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard. Standard Heat Formation Of Copper Oxide.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5773812 Standard Heat Formation Of Copper Oxide For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Copper(ii) oxide permanent link for this species. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Use this link for bookmarking this species for future reference. The standard enthalpy of formation of any element in. Standard Heat Formation Of Copper Oxide.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Standard Heat Formation Of Copper Oxide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Use this link for bookmarking this species for future reference. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. \[\delta h_{reaction}^o = \sum {\delta. The standard enthalpy of formation of any element in its standard. Standard Heat Formation Of Copper Oxide.
From www.researchgate.net
Standard enthalpy of formation from binary oxides vs tolerance factor Standard Heat Formation Of Copper Oxide \[\delta h_{reaction}^o = \sum {\delta. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Use this link for bookmarking this species for future reference. The. Standard Heat Formation Of Copper Oxide.
From www.researchgate.net
Green synthesis route of copper oxide nanoparticles. Download Standard Heat Formation Of Copper Oxide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. \[\delta h_{reaction}^o = \sum {\delta. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. For example, although oxygen can exist as ozone (o 3 ), atomic. Standard Heat Formation Of Copper Oxide.
From readingandwritingprojectcom.web.fc2.com
heat of formation for o2 Standard Heat Formation Of Copper Oxide Copper(ii) oxide permanent link for this species. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Use this link for bookmarking this species for future reference. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard enthalpy change of formation (data table) these tables. Standard Heat Formation Of Copper Oxide.
From pubs.acs.org
Initial Stages of Oxide Formation on Copper Surfaces during Oxygen Standard Heat Formation Of Copper Oxide Copper(ii) oxide permanent link for this species. Use this link for bookmarking this species for future reference. To find the δh reaction o, use the formula for the standard enthalpy change of formation: Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. 193 rows in chemistry and. Standard Heat Formation Of Copper Oxide.
From www.researchgate.net
The growth mechanism of CuO thin films using thermal oxidation. (a Standard Heat Formation Of Copper Oxide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. \[\delta h_{reaction}^o = \sum {\delta. To find the δh reaction o, use the formula for the standard. Standard Heat Formation Of Copper Oxide.
From www.youtube.com
Copper Oxide Synthesis YouTube Standard Heat Formation Of Copper Oxide Copper(ii) oxide permanent link for this species. \[\delta h_{reaction}^o = \sum {\delta. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Use this link for. Standard Heat Formation Of Copper Oxide.
From www.mdpi.com
Applied Mechanics Free FullText Heat Transfer Deterioration by the Standard Heat Formation Of Copper Oxide \[\delta h_{reaction}^o = \sum {\delta. Use this link for bookmarking this species for future reference. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone. Standard Heat Formation Of Copper Oxide.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Standard Heat Formation Of Copper Oxide Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Standard Heat Formation Of Copper Oxide.
From www.semanticscholar.org
Figure 3 from Thermal Oxidation of Copper for Favorable Formation of Standard Heat Formation Of Copper Oxide The standard enthalpy of formation of any element in its standard state is zero by definition. To find the δh reaction o, use the formula for the standard enthalpy change of formation: Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Use this link for bookmarking this. Standard Heat Formation Of Copper Oxide.
From worksheetzonepatine.z14.web.core.windows.net
Heat Of Formation Chart Standard Heat Formation Of Copper Oxide For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. To find the δh reaction o, use the formula for the standard enthalpy change of formation: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element. Standard Heat Formation Of Copper Oxide.
From w20.b2m.cz
Sendo O Delta H De Formação Do óxido De Cobre EDUCA Standard Heat Formation Of Copper Oxide Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Copper(ii) oxide permanent link for this species. To find the δh reaction o, use the formula for the standard enthalpy change of formation: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Heat Formation Of Copper Oxide.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Formation Of Copper Oxide Use this link for bookmarking this species for future reference. Copper(ii) oxide permanent link for this species. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. \[\delta h_{reaction}^o = \sum {\delta. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard. Standard Heat Formation Of Copper Oxide.
From www.youtube.com
Heating a mixture of copper (II) oxide and carbon C0118 YouTube Standard Heat Formation Of Copper Oxide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Copper(ii) oxide permanent link for this species. To find the δh reaction o, use the formula for the. Standard Heat Formation Of Copper Oxide.
From www.researchgate.net
Solgel citrate method for synthesis of Copper oxide Nanoparticles Standard Heat Formation Of Copper Oxide To find the δh reaction o, use the formula for the standard enthalpy change of formation: Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data. Standard Heat Formation Of Copper Oxide.
From www.researchgate.net
Graphical representation of biomediated synthesis of copper oxide Standard Heat Formation Of Copper Oxide Use this link for bookmarking this species for future reference. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. \[\delta h_{reaction}^o = \sum {\delta. Standand enthalpies of formation & standard entropies of common compounds substance. Standard Heat Formation Of Copper Oxide.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Formation Of Copper Oxide Copper(ii) oxide permanent link for this species. Use this link for bookmarking this species for future reference. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. To find the δh reaction o, use the formula for the standard enthalpy change of formation: 193 rows in chemistry and. Standard Heat Formation Of Copper Oxide.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Standard Heat Formation Of Copper Oxide For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Copper(ii) oxide permanent link. Standard Heat Formation Of Copper Oxide.
From www.mdpi.com
Applied Mechanics Free FullText Heat Transfer Deterioration by the Standard Heat Formation Of Copper Oxide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. To find the δh. Standard Heat Formation Of Copper Oxide.
From hxewqbuhm.blob.core.windows.net
Copper Oxide Formation at Ronald Sullivan blog Standard Heat Formation Of Copper Oxide Use this link for bookmarking this species for future reference. \[\delta h_{reaction}^o = \sum {\delta. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist. Standard Heat Formation Of Copper Oxide.
From socratic.org
Using Hess' Law, how do you calculate the standard heat of formation of Standard Heat Formation Of Copper Oxide The standard enthalpy of formation of any element in its standard state is zero by definition. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Use this link for bookmarking this species for future reference. For example, although oxygen can exist as ozone (o 3 ), atomic. Standard Heat Formation Of Copper Oxide.
From www.researchgate.net
FESEM images of copper oxide phases with increasing oxygen flow rate Standard Heat Formation Of Copper Oxide Copper(ii) oxide permanent link for this species. To find the δh reaction o, use the formula for the standard enthalpy change of formation: \[\delta h_{reaction}^o = \sum {\delta. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of. Standard Heat Formation Of Copper Oxide.
From es.slideshare.net
Thermal Oxidation of Copper for Favorable Formation of Cupric Oxide Standard Heat Formation Of Copper Oxide To find the δh reaction o, use the formula for the standard enthalpy change of formation: \[\delta h_{reaction}^o = \sum {\delta. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Copper(ii) oxide permanent link for. Standard Heat Formation Of Copper Oxide.
From www.researchgate.net
Scheme 1. Synthesis of copper oxide nanoparticles (CuO NPs) from waste Standard Heat Formation Of Copper Oxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. \[\delta h_{reaction}^o = \sum {\delta. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Copper(ii) oxide permanent link for this species. Definition and explanation. Standard Heat Formation Of Copper Oxide.
From www.researchgate.net
Schematic presentation of copper oxidecobalt oxide NWs formation Standard Heat Formation Of Copper Oxide Copper(ii) oxide permanent link for this species. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Heat Formation Of Copper Oxide.
From www.slideshare.net
Chapter 4 Standard Heat Formation Of Copper Oxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. To find the δh reaction o, use the formula for the standard enthalpy change of formation: The standard enthalpy of formation of any element in its standard state is zero by definition. \[\delta h_{reaction}^o = \sum {\delta.. Standard Heat Formation Of Copper Oxide.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Heat Formation Of Copper Oxide Copper(ii) oxide permanent link for this species. To find the δh reaction o, use the formula for the standard enthalpy change of formation: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Definition. Standard Heat Formation Of Copper Oxide.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Standard Heat Formation Of Copper Oxide Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. \[\delta h_{reaction}^o = \sum {\delta. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Heat Formation Of Copper Oxide.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Standard Heat Formation Of Copper Oxide Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change. Standard Heat Formation Of Copper Oxide.
From www.youtube.com
Heating a mixture of carbon and copper (II) oxide YouTube Standard Heat Formation Of Copper Oxide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. To find the δh reaction o, use the formula for the standard enthalpy change of formation: Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Copper(ii) oxide. Standard Heat Formation Of Copper Oxide.