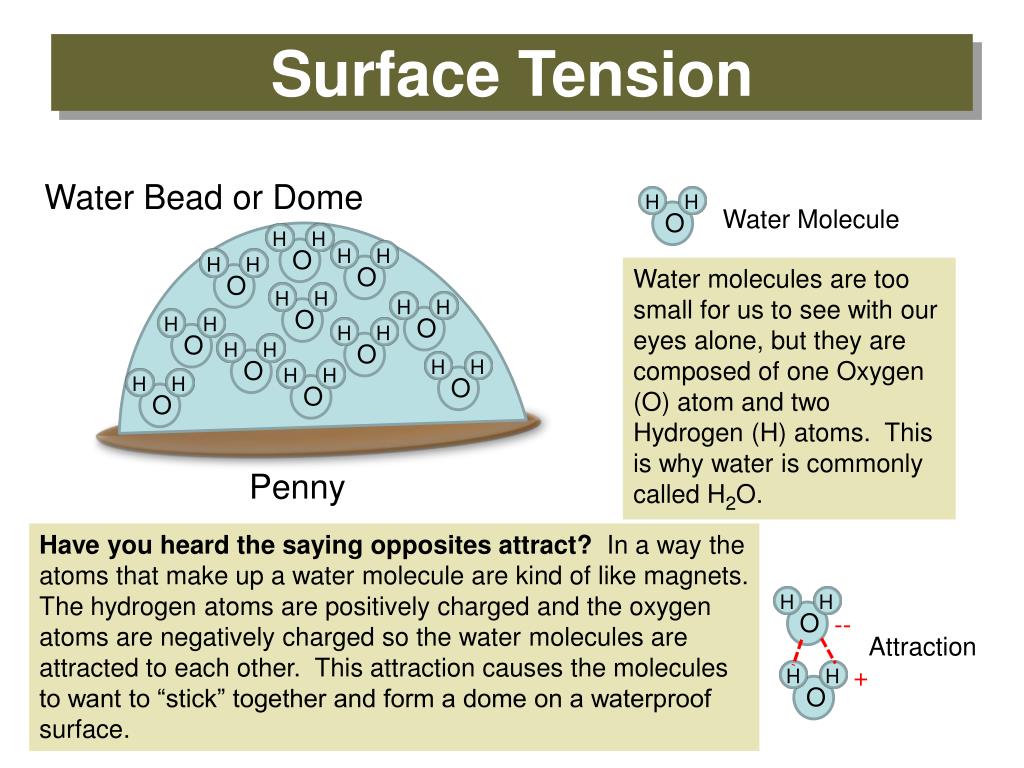
What Is High Water Tension . Since these intermolecular forces vary depending on the nature of the liquid (e.g. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water is 72 dynes/cm at 25°c. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. The surface tension of water decreases significantly with. Surfactants like detergent), each solution exhibits differing surface tension properties. The cohesive forces between liquid molecules are responsible for the. Gasoline) or solutes in the liquid (e.g. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Water consists of one oxygen atom flanked by two hydrogen atoms. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. This term is typically used only. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f).
from www.slideserve.com
Surfactants like detergent), each solution exhibits differing surface tension properties. Since these intermolecular forces vary depending on the nature of the liquid (e.g. It would take a force of 72 dynes to break a surface film of water 1 cm long. The cohesive forces between liquid molecules are responsible for the. This term is typically used only. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Gasoline) or solutes in the liquid (e.g. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,.
PPT Surface Tension PowerPoint Presentation, free download ID3106425
What Is High Water Tension In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. This term is typically used only. Gasoline) or solutes in the liquid (e.g. The surface tension of water is 72 dynes/cm at 25°c. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Water consists of one oxygen atom flanked by two hydrogen atoms. The cohesive forces between liquid molecules are responsible for the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Surfactants like detergent), each solution exhibits differing surface tension properties. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The surface tension of water decreases significantly with.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments What Is High Water Tension The surface tension of water decreases significantly with. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension is a physical property defined as the amount of force required per unit area to expand the. What Is High Water Tension.
From news.mit.edu
MIT research on seawater surface tension international What Is High Water Tension The surface tension of water decreases significantly with. The cohesive forces between liquid molecules are responsible for the. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. It would take a force of 72 dynes to break a surface film of water 1 cm long. The. What Is High Water Tension.
From funsizephysics.com
What is Surface Tension? FunsizePhysics What Is High Water Tension In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists. What Is High Water Tension.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it What Is High Water Tension Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surfactants like detergent), each solution exhibits differing surface tension properties. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a. What Is High Water Tension.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Is High Water Tension The cohesive forces between liquid molecules are responsible for the. Water consists of one oxygen atom flanked by two hydrogen atoms. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The surface tension of water is 72 dynes/cm at 25°c. Surface tension is the energy, or work, required to increase the surface area. What Is High Water Tension.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download What Is High Water Tension It would take a force of 72 dynes to break a surface film of water 1 cm long. Gasoline) or solutes in the liquid (e.g. The cohesive forces between liquid molecules are responsible for the. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Since these. What Is High Water Tension.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID1714027 What Is High Water Tension The cohesive forces between liquid molecules are responsible for the. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by two hydrogen atoms. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as. What Is High Water Tension.
From thehomeschoolscientist.com
Surface Tension In Water Explanation and Experiment The Homeschool What Is High Water Tension Water has high surface tension, which can be explained by its polarity and hydrogen bonding. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. This term is typically used only. Water consists of one oxygen atom flanked by two hydrogen atoms. Surface tension is the energy, or work, required to increase the surface area. What Is High Water Tension.
From hxeafvitb.blob.core.windows.net
Why Water Have High Surface Tension at Nancy Aguirre blog What Is High Water Tension Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Since these intermolecular forces vary depending on the nature of. What Is High Water Tension.
From stock.adobe.com
surface tension wetting surfactant water hydrophilic hydrophobic What Is High Water Tension Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by two hydrogen atoms. Surfactants like detergent), each solution exhibits differing surface tension properties. The surface tension of water is 72 dynes/cm at 25°c. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The. What Is High Water Tension.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Is High Water Tension Water consists of one oxygen atom flanked by two hydrogen atoms. The cohesive forces between liquid molecules are responsible for the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension of water is 72 dynes/cm at 25°c. Surface tension is a phenomenon in which the surface. What Is High Water Tension.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download What Is High Water Tension Surfactants like detergent), each solution exhibits differing surface tension properties. It would take a force of 72 dynes to break a surface film of water 1 cm long. The cohesive forces between liquid molecules are responsible for the. The surface tension of water is 72 dynes/cm at 25°c. Water has high surface tension, which can be explained by its polarity. What Is High Water Tension.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii What Is High Water Tension In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules are responsible for the. This term is typically used only. Gasoline) or solutes in the liquid (e.g. The surface. What Is High Water Tension.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids What Is High Water Tension Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension of water decreases significantly with. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surfactants like detergent), each solution. What Is High Water Tension.
From www.youtube.com
Surface tension of water YouTube What Is High Water Tension Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. The surface tension of water decreases significantly with. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. The surface tension of water is 72 dynes/cm at 25°c. It would take a. What Is High Water Tension.
From ar.inspiredpencil.com
Surface Tension Water What Is High Water Tension Since these intermolecular forces vary depending on the nature of the liquid (e.g. Water consists of one oxygen atom flanked by two hydrogen atoms. Surfactants like detergent), each solution exhibits differing surface tension properties. Gasoline) or solutes in the liquid (e.g. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. The surface tension of. What Is High Water Tension.
From www.vrogue.co
Surface Tension Of Water Why Is It So High vrogue.co What Is High Water Tension Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The surface tension of water is 72 dynes/cm at 25°c. The surface tension of water decreases significantly with. Gasoline) or solutes in the liquid (e.g. This term is typically used only. Water has high surface tension, which can be explained. What Is High Water Tension.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Is High Water Tension Since these intermolecular forces vary depending on the nature of the liquid (e.g. Water consists of one oxygen atom flanked by two hydrogen atoms. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. This term is typically used only. The surface tension of water is 72 dynes/cm at 25°c. Surface tension is a physical. What Is High Water Tension.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces What Is High Water Tension It would take a force of 72 dynes to break a surface film of water 1 cm long. This term is typically used only. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. The surface tension of water is 72 dynes/cm at 25°c. Surface tension is the energy, or work, required to increase the. What Is High Water Tension.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID What Is High Water Tension The cohesive forces between liquid molecules are responsible for the. Since these intermolecular forces vary depending on the nature of the liquid (e.g. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water is 72 dynes/cm at 25°c. Surfactants like detergent), each solution exhibits differing surface tension. What Is High Water Tension.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 What Is High Water Tension Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surfactants like detergent), each solution exhibits differing surface tension properties. Water consists of one oxygen atom flanked by two hydrogen atoms. The surface tension of water is 72 dynes/cm. What Is High Water Tension.
From www.thoughtco.com
Surface Tension Definition in Chemistry What Is High Water Tension The cohesive forces between liquid molecules are responsible for the. Surfactants like detergent), each solution exhibits differing surface tension properties. This term is typically used only. Gasoline) or solutes in the liquid (e.g. The surface tension of water decreases significantly with. The surface tension of water is 72 dynes/cm at 25°c. Surface tension is a phenomenon in which the surface. What Is High Water Tension.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Is High Water Tension Since these intermolecular forces vary depending on the nature of the liquid (e.g. The cohesive forces between liquid molecules are responsible for the. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The. What Is High Water Tension.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download What Is High Water Tension In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Surfactants like detergent), each solution exhibits differing surface tension properties. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Gasoline) or solutes in the liquid (e.g.. What Is High Water Tension.
From chem.libretexts.org
Chapter 7.3 Unique Properties of Liquids Chemistry LibreTexts What Is High Water Tension Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by two hydrogen atoms. The cohesive forces between liquid molecules are responsible for the. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid.. What Is High Water Tension.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? What Is High Water Tension Gasoline) or solutes in the liquid (e.g. Water consists of one oxygen atom flanked by two hydrogen atoms. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68. What Is High Water Tension.
From www.researchgate.net
a wetting state of surface with low and highsurfacetension fluids b What Is High Water Tension Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Surfactants like detergent), each solution exhibits differing surface tension properties. Surface tension is a phenomenon in which the surface of a liquid, where the liquid. What Is High Water Tension.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Is High Water Tension Water consists of one oxygen atom flanked by two hydrogen atoms. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. Since these intermolecular forces vary depending on the nature of the liquid (e.g. It would take a force of 72 dynes to break a surface film of water 1 cm long. Water has high. What Is High Water Tension.
From www.mdpi.com
Symmetry Free FullText ParticleBased Dynamic Water Drops with What Is High Water Tension Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a. What Is High Water Tension.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Is High Water Tension In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. This term is typically used only. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension is the energy, or work, required to increase the surface area of a liquid. What Is High Water Tension.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical What Is High Water Tension The surface tension of water decreases significantly with. The cohesive forces between liquid molecules are responsible for the. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions ,. This term is typically used only. Water consists of one oxygen. What Is High Water Tension.
From hxedgviga.blob.core.windows.net
What Causes High Surface Tension Of Water at Todd James blog What Is High Water Tension Surfactants like detergent), each solution exhibits differing surface tension properties. It would take a force of 72 dynes to break a surface film of water 1 cm long. Gasoline) or solutes in the liquid (e.g. Water consists of one oxygen atom flanked by two hydrogen atoms. In comparison, organic liquids, such as benzene and alcohols , have lower surface tensions. What Is High Water Tension.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse What Is High Water Tension Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surfactants like detergent), each solution exhibits differing surface tension properties. The surface tension of water decreases significantly with. Water has a surface tension of 0.07275. What Is High Water Tension.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Is High Water Tension Since these intermolecular forces vary depending on the nature of the liquid (e.g. The surface tension of water decreases significantly with. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water is 72 dynes/cm at 25°c. Water has a surface tension of 0.07275 joule per square metre. What Is High Water Tension.
From www.youtube.com
Surface Tension of Water Explained YouTube What Is High Water Tension The surface tension of water decreases significantly with. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by two hydrogen atoms. The surface tension of water is 72 dynes/cm at 25°c.. What Is High Water Tension.