What Is The Meaning Of K In The Expression Below . The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, k c. The general equilibrium expression for a reaction: The equilibrium constant is the value of the reaction quotient that is calculated from the expression for chemical equilibrium. The brackets [ ] represent the. The equilibrium constant for a reaction that is the sum of two or more reactions is equal to the product of the equilibrium constants for the. It assumes that you are familiar with the. K has a unique value for a given reaction at a fixed temperature and. The equilibrium constant k is the value of q when the reaction is at equilibrium. It depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in a solution. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. Writing expressions for k c.
from www.storyofmathematics.com
The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. The equilibrium constant k is the value of q when the reaction is at equilibrium. K has a unique value for a given reaction at a fixed temperature and. It depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in a solution. It assumes that you are familiar with the. The brackets [ ] represent the. This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, k c. The equilibrium constant for a reaction that is the sum of two or more reactions is equal to the product of the equilibrium constants for the. The equilibrium constant is the value of the reaction quotient that is calculated from the expression for chemical equilibrium. The general equilibrium expression for a reaction:
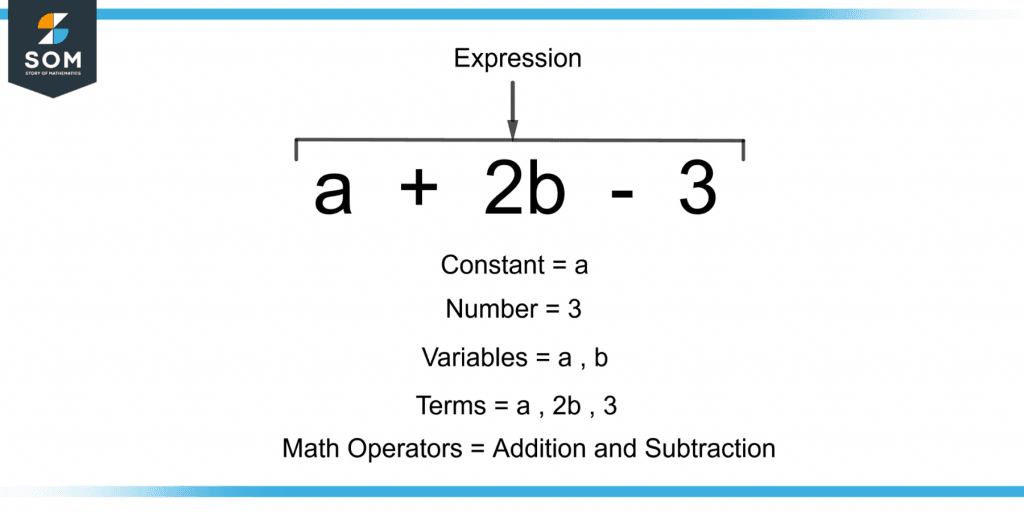
Expression Definition & Meaning
What Is The Meaning Of K In The Expression Below The equilibrium constant k is the value of q when the reaction is at equilibrium. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The equilibrium constant is the value of the reaction quotient that is calculated from the expression for chemical equilibrium. K has a unique value for a given reaction at a fixed temperature and. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. Writing expressions for k c. It depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in a solution. The general equilibrium expression for a reaction: The brackets [ ] represent the. This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, k c. It assumes that you are familiar with the. The equilibrium constant k is the value of q when the reaction is at equilibrium. The equilibrium constant for a reaction that is the sum of two or more reactions is equal to the product of the equilibrium constants for the.
From hinative.com
What is the meaning of "what is K mean? 1k 10k 100k What Is The Meaning Of K In The Expression Below The general equilibrium expression for a reaction: The equilibrium constant is the value of the reaction quotient that is calculated from the expression for chemical equilibrium. The brackets [ ] represent the. It assumes that you are familiar with the. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the. What Is The Meaning Of K In The Expression Below.
From www.youtube.com
Writing K expressions YouTube What Is The Meaning Of K In The Expression Below K has a unique value for a given reaction at a fixed temperature and. The equilibrium constant is the value of the reaction quotient that is calculated from the expression for chemical equilibrium. The general equilibrium expression for a reaction: The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a.. What Is The Meaning Of K In The Expression Below.
From www.doubtnut.com
Explain the meaning of K.E. with examples. Obtain an expression for K What Is The Meaning Of K In The Expression Below This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, k c. The general equilibrium expression for a reaction: It depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in a solution. It assumes that you are familiar with the. The equilibrium constant is. What Is The Meaning Of K In The Expression Below.
From www.storyofmathematics.com
Expression Definition & Meaning What Is The Meaning Of K In The Expression Below The equilibrium constant is the value of the reaction quotient that is calculated from the expression for chemical equilibrium. The equilibrium constant for a reaction that is the sum of two or more reactions is equal to the product of the equilibrium constants for the. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at. What Is The Meaning Of K In The Expression Below.
From www.youtube.com
k se word meaning k se 50+ word meaning k se meaning K se What Is The Meaning Of K In The Expression Below The equilibrium constant for a reaction that is the sum of two or more reactions is equal to the product of the equilibrium constants for the. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. It depends on the ionic strength and temperature and is independent of the concentrations. What Is The Meaning Of K In The Expression Below.
From www.youtube.com
Equilibrium 7 The Meaning of K YouTube What Is The Meaning Of K In The Expression Below It depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in a solution. It assumes that you are familiar with the. The equilibrium constant for a reaction that is the sum of two or more reactions is equal to the product of the equilibrium constants for the. The equilibrium constant k is. What Is The Meaning Of K In The Expression Below.
From www.youtube.com
Understanding Ka and Kb Expressions YouTube What Is The Meaning Of K In The Expression Below The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. It assumes that you are familiar with the. Writing expressions for k c. The equilibrium constant is the value of the reaction quotient that is calculated from the expression for chemical equilibrium. K has a unique value for a given. What Is The Meaning Of K In The Expression Below.
From www.chegg.com
Solved 1. [3] For which values of the constant k is the What Is The Meaning Of K In The Expression Below The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations,. What Is The Meaning Of K In The Expression Below.
From www.cuemath.com
Expression Term, Factor, Coefficient Definition and examples Cuemath What Is The Meaning Of K In The Expression Below The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The brackets [ ] represent the. The equilibrium constant is the value of the reaction quotient that is calculated from the expression for chemical equilibrium. K has a unique value for a given reaction at a fixed temperature. What Is The Meaning Of K In The Expression Below.
From www.youtube.com
Find the Value of k in Quadratic Equations when One Root is Given What Is The Meaning Of K In The Expression Below It depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in a solution. Writing expressions for k c. The brackets [ ] represent the. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. K has a unique value for. What Is The Meaning Of K In The Expression Below.
From www.youtube.com
Expression Meaning of expression YouTube What Is The Meaning Of K In The Expression Below The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. It assumes that you are familiar with the. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. The equilibrium constant for a reaction that is the sum. What Is The Meaning Of K In The Expression Below.
From byjus.com
Expression and derivation of equivalent thermal conductivity K in What Is The Meaning Of K In The Expression Below The general equilibrium expression for a reaction: The brackets [ ] represent the. It assumes that you are familiar with the. It depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in a solution. This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations,. What Is The Meaning Of K In The Expression Below.
From www.geeksforgeeks.org
What is an Expression and What are the types of Expressions What Is The Meaning Of K In The Expression Below The equilibrium constant is the value of the reaction quotient that is calculated from the expression for chemical equilibrium. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The brackets [ ] represent the. The general equilibrium expression for a reaction: The equilibrium constant k is the. What Is The Meaning Of K In The Expression Below.
From www.slideserve.com
PPT Chapter 16 Chemical Equilibria PowerPoint Presentation, free What Is The Meaning Of K In The Expression Below It assumes that you are familiar with the. The general equilibrium expression for a reaction: The equilibrium constant k is the value of q when the reaction is at equilibrium. The equilibrium constant is the value of the reaction quotient that is calculated from the expression for chemical equilibrium. The equilibrium constant of a chemical reaction (usually denoted by the. What Is The Meaning Of K In The Expression Below.
From mavink.com
K Means Cluster Diagram What Is The Meaning Of K In The Expression Below This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, k c. The general equilibrium expression for a reaction: It depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in a solution. Writing expressions for k c. The equilibrium constant, k, expresses the relationship. What Is The Meaning Of K In The Expression Below.
From www.youtube.com
Class 12 Maths Lecture 32 Chapter 3 Introduction to Kmethod What Is The Meaning Of K In The Expression Below Writing expressions for k c. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. It depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in a solution. The brackets [ ] represent the. The equilibrium constant is the value of the. What Is The Meaning Of K In The Expression Below.
From www.geeksforgeeks.org
What is an Expression and What are the types of Expressions What Is The Meaning Of K In The Expression Below The brackets [ ] represent the. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The equilibrium constant k is the value of q when the reaction is at equilibrium. It depends on the ionic strength and temperature and is independent of the concentrations of reactants and. What Is The Meaning Of K In The Expression Below.
From www.researchgate.net
Definition of kcore, kshells and maximum kcore. Download What Is The Meaning Of K In The Expression Below Writing expressions for k c. This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, k c. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. The equilibrium constant is the value of the reaction quotient that is calculated from the. What Is The Meaning Of K In The Expression Below.
From www.slideserve.com
PPT ANALYTICAL CHEMISTRY PowerPoint Presentation, free download ID What Is The Meaning Of K In The Expression Below K has a unique value for a given reaction at a fixed temperature and. It assumes that you are familiar with the. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The brackets [ ] represent the. The equilibrium constant is the value of the reaction quotient. What Is The Meaning Of K In The Expression Below.
From www.youtube.com
Write the expression for the equilibrium constant, Kc for each of the What Is The Meaning Of K In The Expression Below Writing expressions for k c. This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, k c. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. The general equilibrium expression for a reaction: It assumes that you are familiar with the.. What Is The Meaning Of K In The Expression Below.
From www.slideserve.com
PPT 11 Writing Expressions and Equations PowerPoint Presentation What Is The Meaning Of K In The Expression Below The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. Writing expressions for k c. The equilibrium constant for a reaction that is the sum of two or more reactions is equal to the product of the equilibrium constants for the. The general equilibrium expression for a reaction: It assumes. What Is The Meaning Of K In The Expression Below.
From www.showme.com
The Chemists Toolbox The Meaning of K Science, Chemistry ShowMe What Is The Meaning Of K In The Expression Below The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. The equilibrium constant for a reaction that is the sum of two or more reactions is equal to the product of the equilibrium constants for the. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides. What Is The Meaning Of K In The Expression Below.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free What Is The Meaning Of K In The Expression Below Writing expressions for k c. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. The equilibrium constant k is the value of q when the reaction is at equilibrium. This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, k c.. What Is The Meaning Of K In The Expression Below.
From learn.co
Programming Univbasics Expression And... Learn.co What Is The Meaning Of K In The Expression Below The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. The general equilibrium expression for a reaction: It assumes that you are familiar with the. This page explains. What Is The Meaning Of K In The Expression Below.
From www.slideserve.com
PPT Chapter 16 Principles of Reactivity Chemical Equilibria What Is The Meaning Of K In The Expression Below The general equilibrium expression for a reaction: K has a unique value for a given reaction at a fixed temperature and. This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, k c. It depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in. What Is The Meaning Of K In The Expression Below.
From grammarhow.com
What Does 'K' Mean in Money? (Helpful Examples) What Is The Meaning Of K In The Expression Below It depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in a solution. The equilibrium constant k is the value of q when the reaction is at equilibrium. The equilibrium constant is the value of the reaction quotient that is calculated from the expression for chemical equilibrium. The brackets [ ] represent. What Is The Meaning Of K In The Expression Below.
From lessonlangdonobang.z21.web.core.windows.net
What Is K In An Equation What Is The Meaning Of K In The Expression Below K has a unique value for a given reaction at a fixed temperature and. Writing expressions for k c. The equilibrium constant k is the value of q when the reaction is at equilibrium. The equilibrium constant for a reaction that is the sum of two or more reactions is equal to the product of the equilibrium constants for the.. What Is The Meaning Of K In The Expression Below.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID What Is The Meaning Of K In The Expression Below The equilibrium constant k is the value of q when the reaction is at equilibrium. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. Writing expressions for. What Is The Meaning Of K In The Expression Below.
From eslforums.com
Words that Start with K List of 955+ Common K Words with ESL Pictures What Is The Meaning Of K In The Expression Below The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. Writing expressions for k c. The equilibrium constant for a reaction that is the sum of two or more reactions is equal to the product of the equilibrium constants for the. The equilibrium constant is the value of the reaction. What Is The Meaning Of K In The Expression Below.
From www.chegg.com
Solved Write the expression for K for the reaction below. What Is The Meaning Of K In The Expression Below The brackets [ ] represent the. K has a unique value for a given reaction at a fixed temperature and. This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, k c. The equilibrium constant for a reaction that is the sum of two or more reactions is equal to the product. What Is The Meaning Of K In The Expression Below.
From www.is.com
What is KFactor for Apps and How Can You Increase It? ironSource What Is The Meaning Of K In The Expression Below The equilibrium constant for a reaction that is the sum of two or more reactions is equal to the product of the equilibrium constants for the. It depends on the ionic strength and temperature and is independent of the concentrations of reactants and products in a solution. It assumes that you are familiar with the. The equilibrium constant k is. What Is The Meaning Of K In The Expression Below.
From legal-explanations.com
K Definition What Does K Mean? What Is The Meaning Of K In The Expression Below The equilibrium constant k is the value of q when the reaction is at equilibrium. Writing expressions for k c. This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, k c. The general equilibrium expression for a reaction: It depends on the ionic strength and temperature and is independent of the. What Is The Meaning Of K In The Expression Below.
From www.youtube.com
Writing Equilibrium Expressions YouTube What Is The Meaning Of K In The Expression Below This page explains what is meant by an equilibrium constant, introducing equilibrium constants expressed in terms of concentrations, k c. Writing expressions for k c. K has a unique value for a given reaction at a fixed temperature and. It assumes that you are familiar with the. It depends on the ionic strength and temperature and is independent of the. What Is The Meaning Of K In The Expression Below.
From s.mriquestions.com
kspace Questions and Answers in MRI What Is The Meaning Of K In The Expression Below The equilibrium constant k is the value of q when the reaction is at equilibrium. The brackets [ ] represent the. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The equilibrium constant for a reaction that is the sum of two or more reactions is equal. What Is The Meaning Of K In The Expression Below.
From www.youtube.com
Find the Value of K to make a Perfect Square Trinomial StepbyStep What Is The Meaning Of K In The Expression Below The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. The brackets [ ] represent the. Writing expressions for k c. The equilibrium constant k is the value of q when the reaction is at equilibrium. K has a unique value for a given reaction at a fixed temperature and.. What Is The Meaning Of K In The Expression Below.