Positive Electrode Definition In Chemistry . Interpret electrode potentials in terms of relative oxidant and reductant strengths. — if the voltmeter is removed and replaced with a bulb or if the circuit is short circuited, a current flows. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,. describe and relate the definitions of electrode and cell potentials. — a wire connects the two reacting substances. The flow of electrons through that wire is electricity. — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. The battery’s negative electrode is the.
from www.fdk.com
— a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The battery’s negative electrode is the. — a wire connects the two reacting substances. — if the voltmeter is removed and replaced with a bulb or if the circuit is short circuited, a current flows. The flow of electrons through that wire is electricity. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,.
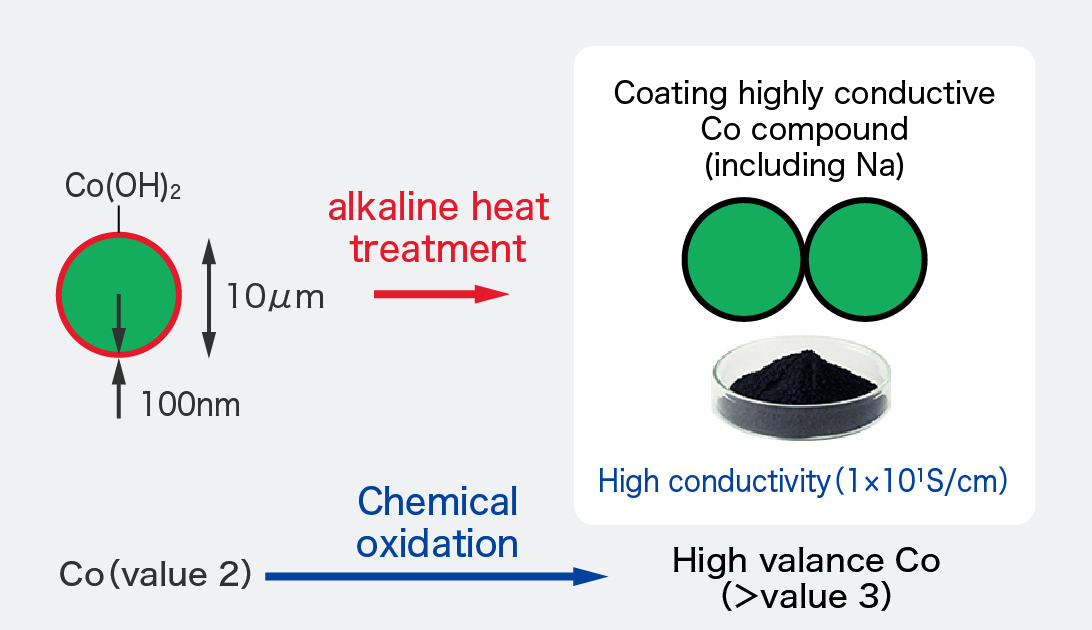
Positive electrode material │ FDK's original technology │ R&D │ FDK
Positive Electrode Definition In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant strengths. The battery’s negative electrode is the. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. — a wire connects the two reacting substances. — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. describe and relate the definitions of electrode and cell potentials. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The flow of electrons through that wire is electricity. — if the voltmeter is removed and replaced with a bulb or if the circuit is short circuited, a current flows. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? Positive Electrode Definition In Chemistry The battery’s negative electrode is the. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The flow of electrons through that wire is electricity. — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. — a wire connects the two reacting substances. — the electrode attached to. Positive Electrode Definition In Chemistry.
From www.aakash.ac.in
Standard Electrode Potential Importance, Definition & Redox Reactions Positive Electrode Definition In Chemistry The flow of electrons through that wire is electricity. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,. The battery’s negative electrode is the. — a wire connects the two reacting substances. — a simple type of electrode reaction involves only electron. Positive Electrode Definition In Chemistry.
From dxontbmym.blob.core.windows.net
Electrode Meaning Chemistry at Christy Rogers blog Positive Electrode Definition In Chemistry The flow of electrons through that wire is electricity. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,. — a wire connects the two reacting substances. — if the voltmeter is removed and replaced with a bulb or if the circuit is. Positive Electrode Definition In Chemistry.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry Positive Electrode Definition In Chemistry — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. describe and relate the definitions of electrode and cell potentials. — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. The battery’s negative electrode is the. — if the voltmeter is removed and. Positive Electrode Definition In Chemistry.
From www.researchgate.net
Figure1. Diagrams of positive electrode active mass of the Positive Electrode Definition In Chemistry The battery’s negative electrode is the. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. The flow of electrons through that wire is electricity. describe and relate the definitions of electrode and cell. Positive Electrode Definition In Chemistry.
From www.fdk.com
Positive electrode material │ FDK's original technology │ R&D │ FDK Positive Electrode Definition In Chemistry describe and relate the definitions of electrode and cell potentials. The flow of electrons through that wire is electricity. Interpret electrode potentials in terms of relative oxidant and reductant strengths. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. — a wire connects the two reacting substances. — the electrode. Positive Electrode Definition In Chemistry.
From byjus.com
What is an active electrode? Explain with the help of an example Positive Electrode Definition In Chemistry — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. describe and relate the definitions of electrode and cell potentials. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,. — the electrode attached. Positive Electrode Definition In Chemistry.
From mavink.com
Electrode Classification Diagram Positive Electrode Definition In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant strengths. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The battery’s negative electrode is the. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. — the potential of an electrochemical cell. Positive Electrode Definition In Chemistry.
From www.researchgate.net
(PDF) Theoretical picture of positive electrode / solid electrolyte Positive Electrode Definition In Chemistry — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Interpret electrode potentials in terms of relative oxidant and reductant strengths. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. — if the voltmeter is removed and replaced with a bulb or if. Positive Electrode Definition In Chemistry.
From study.com
Electrode Definition, Types & Function Lesson Positive Electrode Definition In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant strengths. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. The battery’s negative electrode is the. — a wire connects the two reacting substances. The flow of electrons through that wire is electricity. — if the voltmeter is removed and replaced. Positive Electrode Definition In Chemistry.
From exoxpbgzu.blob.core.windows.net
Define Electrodes In Chemistry Terms at Monte Cordell blog Positive Electrode Definition In Chemistry — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The battery’s negative electrode is the. describe and relate the definitions of electrode and cell potentials. — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. The flow of electrons through. Positive Electrode Definition In Chemistry.
From usermanualtractors.z1.web.core.windows.net
Cathode In Electrochemical Cell Positive Electrode Definition In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant strengths. — if the voltmeter is removed and replaced with a bulb or if the circuit is short circuited, a current flows. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,. —. Positive Electrode Definition In Chemistry.
From www.youtube.com
Electrochemistry 6 Type of Electrodes YouTube Positive Electrode Definition In Chemistry — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,. The flow of electrons through that wire is electricity. — if the voltmeter is removed and replaced with a bulb or if the circuit is short circuited, a current flows. describe and relate. Positive Electrode Definition In Chemistry.
From www.slideserve.com
PPT Chapter 7 Electrolyte solution PowerPoint Presentation, free Positive Electrode Definition In Chemistry — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. The flow of electrons through that wire is electricity. describe and relate the definitions of electrode and cell potentials. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The battery’s negative electrode is. Positive Electrode Definition In Chemistry.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Positive Electrode Definition In Chemistry — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. The battery’s negative electrode is the. The flow of electrons through that wire is electricity. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. — the potential of an electrochemical cell is the. Positive Electrode Definition In Chemistry.
From www.youtube.com
Electrolysis inert electrode O level chemistry by Ms Chew YouTube Positive Electrode Definition In Chemistry — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. — a wire connects the two reacting substances. Interpret electrode potentials in terms of relative oxidant and reductant strengths. —. Positive Electrode Definition In Chemistry.
From www.alamy.com
Positive electrode Stock Vector Images Alamy Positive Electrode Definition In Chemistry — a wire connects the two reacting substances. The battery’s negative electrode is the. describe and relate the definitions of electrode and cell potentials. The flow of electrons through that wire is electricity. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,.. Positive Electrode Definition In Chemistry.
From circuitdbclicheed.z13.web.core.windows.net
Electrochemical Cell Diagram Positive Electrode Definition In Chemistry — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. The battery’s negative electrode is the. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The flow of electrons through that wire is electricity. — the electrode attached to the negative terminal of a battery is called a negative electrode, or. Positive Electrode Definition In Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Positive Electrode Definition In Chemistry The flow of electrons through that wire is electricity. — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. — the positive electrode, on the other hand, will attract negative ions. Positive Electrode Definition In Chemistry.
From www.researchgate.net
Schematic of Liion cell comprising three regions positive electrode Positive Electrode Definition In Chemistry — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. The battery’s negative electrode is the. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,. — a wire connects the two reacting substances. The flow of electrons. Positive Electrode Definition In Chemistry.
From www.fdk.com
Positive electrode material │ FDK's original technology │ R&D │ FDK Positive Electrode Definition In Chemistry — a wire connects the two reacting substances. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,. — a simple type of electrode reaction involves only. Positive Electrode Definition In Chemistry.
From question.pandai.org
Standard Electrode Potential Positive Electrode Definition In Chemistry — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The battery’s negative electrode is the. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,. Interpret electrode potentials in terms of relative oxidant and reductant. Positive Electrode Definition In Chemistry.
From in.pinterest.com
Electrode Potential Energy Definition, Formula and Example Potential Positive Electrode Definition In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant strengths. The battery’s negative electrode is the. — a wire connects the two reacting substances. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. — the electrode attached to the negative terminal of a battery is called a negative electrode, or. Positive Electrode Definition In Chemistry.
From mungfali.com
Reference Electrode Diagram Positive Electrode Definition In Chemistry The battery’s negative electrode is the. The flow of electrons through that wire is electricity. Interpret electrode potentials in terms of relative oxidant and reductant strengths. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. — a wire connects the two reacting substances. — a simple type of electrode reaction involves. Positive Electrode Definition In Chemistry.
From www.theknowledgelibrary.in
Difference Between Cation & Anion The Knowledge Library Positive Electrode Definition In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant strengths. — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. The flow of electrons through that wire is electricity. The battery’s negative electrode is. Positive Electrode Definition In Chemistry.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an Positive Electrode Definition In Chemistry — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. — the positive electrode, on the other hand, will attract negative ions (anions) toward itself. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. — a wire connects the. Positive Electrode Definition In Chemistry.
From circuitagolopetzin.z4.web.core.windows.net
What Happens At The Cathode In Electrolysis Positive Electrode Definition In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant strengths. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. — if the voltmeter. Positive Electrode Definition In Chemistry.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Positive Electrode Definition In Chemistry — a wire connects the two reacting substances. — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. Interpret electrode potentials in terms of relative oxidant and reductant strengths. describe and relate the definitions of electrode and cell potentials. The flow of electrons through that wire is electricity. . Positive Electrode Definition In Chemistry.
From www.chemistry-teaching-resources.com
chemistry picture Positive Electrode Definition In Chemistry Interpret electrode potentials in terms of relative oxidant and reductant strengths. The battery’s negative electrode is the. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode,. Positive Electrode Definition In Chemistry.
From www.nagwa.com
Question Video Identifying the Positive Electrode of a Galvanic Cell Positive Electrode Definition In Chemistry The flow of electrons through that wire is electricity. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. describe and relate the definitions of electrode and cell potentials. — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. —. Positive Electrode Definition In Chemistry.
From www.quinden.co
electrode positif anode et cathode définition Schleun Positive Electrode Definition In Chemistry — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Interpret electrode potentials in terms of relative oxidant and reductant strengths. — a simple type. Positive Electrode Definition In Chemistry.
From www.snexplores.org
Explainer What is an electrode? Positive Electrode Definition In Chemistry — the potential of an electrochemical cell is the difference between the potential at the cathode, ecathode, and the potential at the anode, eanode,. describe and relate the definitions of electrode and cell potentials. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. — a simple type. Positive Electrode Definition In Chemistry.
From de.switch-case.com
Positive oder negative Anode/Kathode in der elektrolytischen Positive Electrode Definition In Chemistry — if the voltmeter is removed and replaced with a bulb or if the circuit is short circuited, a current flows. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. describe and relate the definitions of electrode and cell potentials. — a simple type of electrode reaction. Positive Electrode Definition In Chemistry.
From exoxpbgzu.blob.core.windows.net
Define Electrodes In Chemistry Terms at Monte Cordell blog Positive Electrode Definition In Chemistry The battery’s negative electrode is the. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. — a wire connects the two reacting substances. — if the voltmeter is removed and replaced with a bulb or if the circuit is short circuited, a current flows. describe and relate. Positive Electrode Definition In Chemistry.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 4 electrode half Positive Electrode Definition In Chemistry The battery’s negative electrode is the. — a simple type of electrode reaction involves only electron transfer between an inert metal electrode and an. — the electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The flow of electrons through that wire is electricity. — a wire connects the two. Positive Electrode Definition In Chemistry.