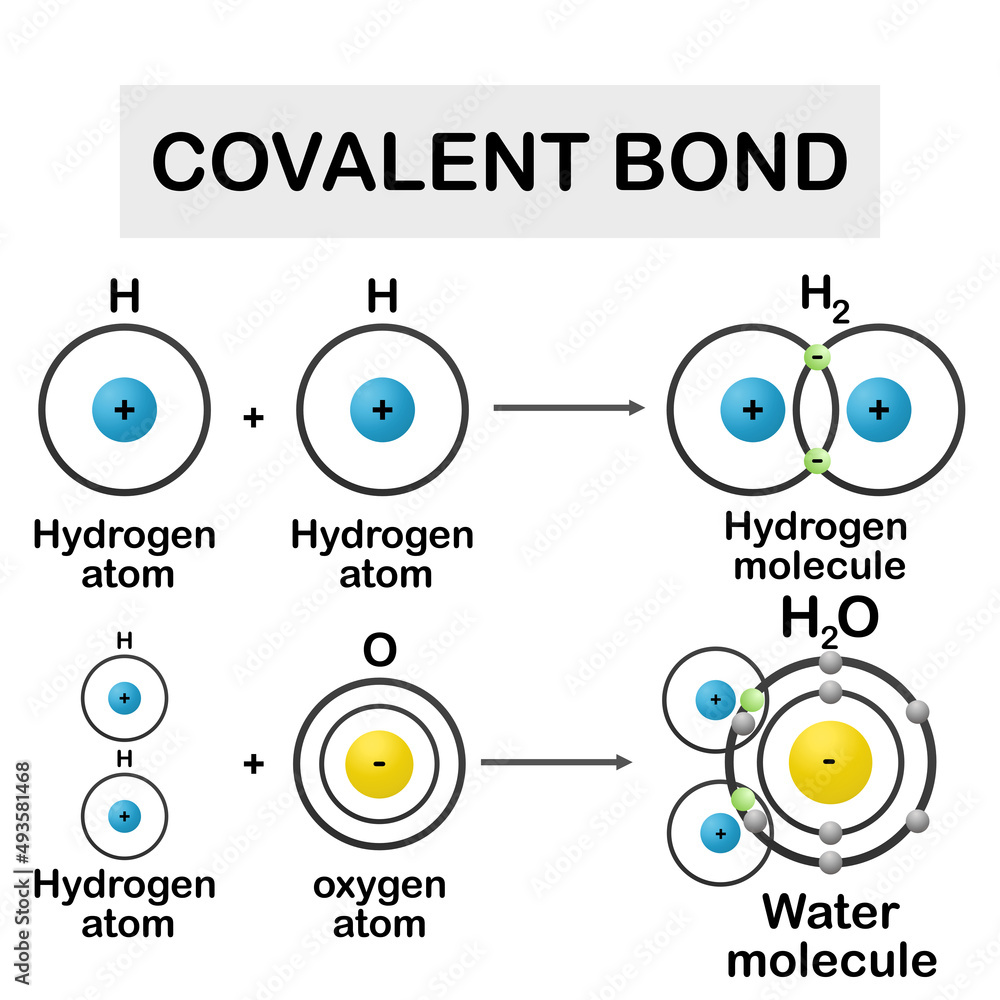
Covalent Model Of Bonding . Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. The shared pair of electrons provides each. This model originated with the theory developed by g.n. Each model has its strengths and weaknesses, and chemists. Covalent bonding occurs when pairs of electrons are shared by atoms. In pure covalent bonds, the. The diatomic hydrogen molecule (h 2) is the simplest model of a covalent bond, and is represented in lewis structures as: In the simplest model (figure 1a) a covalent bond is represented by a straight line between the atoms shown by their element symbols. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. Valence bond theory and molecular orbital theory. Two models have been developed to describe covalent bonding:
from stock.adobe.com
Valence bond theory and molecular orbital theory. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. The shared pair of electrons provides each. Two models have been developed to describe covalent bonding: Each model has its strengths and weaknesses, and chemists. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. In the simplest model (figure 1a) a covalent bond is represented by a straight line between the atoms shown by their element symbols. This model originated with the theory developed by g.n. The diatomic hydrogen molecule (h 2) is the simplest model of a covalent bond, and is represented in lewis structures as: Covalent bonding occurs when pairs of electrons are shared by atoms.
Isolated Covalent bond on white background.Vector illustration.chemical
Covalent Model Of Bonding The shared pair of electrons provides each. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. Valence bond theory and molecular orbital theory. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. The shared pair of electrons provides each. In pure covalent bonds, the. In the simplest model (figure 1a) a covalent bond is represented by a straight line between the atoms shown by their element symbols. This model originated with the theory developed by g.n. Two models have been developed to describe covalent bonding: Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. Covalent bonding occurs when pairs of electrons are shared by atoms. The diatomic hydrogen molecule (h 2) is the simplest model of a covalent bond, and is represented in lewis structures as: Each model has its strengths and weaknesses, and chemists.
From www.tec-science.com
Covalent bonding tecscience Covalent Model Of Bonding Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. The diatomic hydrogen molecule (h 2) is the simplest model of a covalent bond, and is represented in lewis structures as: In the simplest model (figure 1a) a covalent bond is represented by a straight line between the atoms shown. Covalent Model Of Bonding.
From www.teachoo.com
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds Covalent Model Of Bonding The diatomic hydrogen molecule (h 2) is the simplest model of a covalent bond, and is represented in lewis structures as: In pure covalent bonds, the. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. Two models have been developed to describe covalent bonding: Covalent bonds form when electrons. Covalent Model Of Bonding.
From www.msjchem.com
Structure 2.2 The covalent model MSJChem Tutorial videos for IB Covalent Model Of Bonding Two models have been developed to describe covalent bonding: This model originated with the theory developed by g.n. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. The. Covalent Model Of Bonding.
From mmerevise.co.uk
Covalent Bonding Questions and Revision MME Covalent Model Of Bonding Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. In pure covalent bonds, the. This model originated with the theory developed by g.n. Two models have been developed to describe covalent bonding: Each model has its strengths and weaknesses, and chemists. Valence bond theory and molecular. Covalent Model Of Bonding.
From unacademy.com
Covalent Bonding in Carbon Atom Covalent Model Of Bonding The diatomic hydrogen molecule (h 2) is the simplest model of a covalent bond, and is represented in lewis structures as: Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to. Covalent Model Of Bonding.
From www.youtube.com
MODEL OF COVALENT COMPOUNDS(COVALENT BONDING))CLASS TENTHSCIENCE Covalent Model Of Bonding Covalent bonding occurs when pairs of electrons are shared by atoms. Each model has its strengths and weaknesses, and chemists. The diatomic hydrogen molecule (h 2) is the simplest model of a covalent bond, and is represented in lewis structures as: In the simplest model (figure 1a) a covalent bond is represented by a straight line between the atoms shown. Covalent Model Of Bonding.
From cikguwong.blogspot.com
EduMission Chemistry Form 4 Chapter 5 Covalent Bond Covalent Model Of Bonding This model originated with the theory developed by g.n. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. Covalent bonding occurs when pairs of electrons are shared by atoms. In pure covalent bonds, the. The diatomic hydrogen molecule (h 2) is the simplest model of a covalent bond,. Covalent Model Of Bonding.
From www.sliderbase.com
Covalent Bonding (Molecules) Presentation Chemistry Covalent Model Of Bonding Two models have been developed to describe covalent bonding: Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Each model has its strengths and weaknesses, and chemists. In the simplest model (figure 1a) a covalent bond is represented by a straight line between the atoms shown by their element symbols. This. Covalent Model Of Bonding.
From learninglab.rmit.edu.au
Covalent bonds Learning Lab Covalent Model Of Bonding Each model has its strengths and weaknesses, and chemists. In the simplest model (figure 1a) a covalent bond is represented by a straight line between the atoms shown by their element symbols. Valence bond theory and molecular orbital theory. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. Ionic. Covalent Model Of Bonding.
From edu.rsc.org
How to teach covalent bonding CPD RSC Education Covalent Model Of Bonding This model originated with the theory developed by g.n. Each model has its strengths and weaknesses, and chemists. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Covalent bonding occurs when. Covalent Model Of Bonding.
From www.britannica.com
covalent bond Definition, Properties, Examples, & Facts Britannica Covalent Model Of Bonding Two models have been developed to describe covalent bonding: Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. Valence bond theory and molecular orbital theory. Covalent bonding occurs when pairs of electrons are shared by atoms. In the simplest model (figure 1a) a covalent bond is represented by. Covalent Model Of Bonding.
From www.teachit.co.uk
Covalent & ionic bonding models KS4 chemistry Teachit Covalent Model Of Bonding Two models have been developed to describe covalent bonding: Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. The shared pair of electrons provides each. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms.. Covalent Model Of Bonding.
From stock.adobe.com
Isolated Covalent bond on white background.Vector illustration.chemical Covalent Model Of Bonding This model originated with the theory developed by g.n. The shared pair of electrons provides each. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Valence bond theory and molecular. Covalent Model Of Bonding.
From mavink.com
Covalent Bond Types Covalent Model Of Bonding Valence bond theory and molecular orbital theory. Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. The diatomic hydrogen molecule (h 2) is the simplest model of a covalent bond, and is represented in lewis structures as: Atoms will covalently bond with other atoms in order. Covalent Model Of Bonding.
From www.youtube.com
Covalent Bonding DotCross Diagrams GCSE Chemistry Revision YouTube Covalent Model Of Bonding In pure covalent bonds, the. Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. In the simplest model (figure 1a) a covalent bond is represented by a straight line between the atoms shown by their element symbols. Covalent bonding occurs when pairs of electrons are shared. Covalent Model Of Bonding.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Covalent Model Of Bonding Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. In pure covalent bonds, the. Two models have been developed to describe covalent bonding: Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. In the simplest model (figure. Covalent Model Of Bonding.
From www.breakingatom.com
Covalent Bonding Covalent Model Of Bonding Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. The shared pair of electrons provides each. In the simplest model (figure 1a) a covalent bond is represented by a straight line between the atoms shown by their element symbols. Atoms will covalently bond with other atoms in order. Covalent Model Of Bonding.
From saylordotorg.github.io
Molecular Geometry and Covalent Bonding Models Covalent Model Of Bonding Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. In pure covalent bonds, the. The shared pair of electrons provides each. The diatomic hydrogen molecule. Covalent Model Of Bonding.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities Covalent Model Of Bonding The shared pair of electrons provides each. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. In the simplest model (figure 1a) a covalent bond is represented by a straight line between the atoms shown by their element symbols. In pure covalent bonds, the. Covalent bonds are formed between two atoms. Covalent Model Of Bonding.
From www.istockphoto.com
Structure Of Covalent Bond On White Background Stock Illustration Covalent Model Of Bonding Two models have been developed to describe covalent bonding: Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. The diatomic hydrogen molecule (h 2) is the simplest model of a covalent. Covalent Model Of Bonding.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Covalent Model Of Bonding Two models have been developed to describe covalent bonding: Each model has its strengths and weaknesses, and chemists. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. This model originated with the theory developed by g.n. Covalent bonds are formed between two atoms when both have similar tendencies to. Covalent Model Of Bonding.
From bio1151b.nicerweb.net
covalent_bond.html 02_11FourCovalentBonds_A.jpg Covalent Model Of Bonding Covalent bonding occurs when pairs of electrons are shared by atoms. The shared pair of electrons provides each. Valence bond theory and molecular orbital theory. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. Two models have been developed to describe covalent bonding: This model originated with the. Covalent Model Of Bonding.
From www.thoughtco.com
Examples of Covalent Bonds and Compounds Covalent Model Of Bonding This model originated with the theory developed by g.n. In the simplest model (figure 1a) a covalent bond is represented by a straight line between the atoms shown by their element symbols. Two models have been developed to describe covalent bonding: Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Ionic. Covalent Model Of Bonding.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID4132371 Covalent Model Of Bonding The diatomic hydrogen molecule (h 2) is the simplest model of a covalent bond, and is represented in lewis structures as: Covalent bonding occurs when pairs of electrons are shared by atoms. Valence bond theory and molecular orbital theory. The shared pair of electrons provides each. Covalent bonds form when electrons are shared between atoms and are attracted by the. Covalent Model Of Bonding.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples Covalent Model Of Bonding Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. In. Covalent Model Of Bonding.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Covalent Model Of Bonding Covalent bonding occurs when pairs of electrons are shared by atoms. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. Two models have been developed to describe covalent bonding: Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a. Covalent Model Of Bonding.
From joisqugyk.blob.core.windows.net
Models Of Covalent Bonding at Alyce Smith blog Covalent Model Of Bonding Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. In pure covalent bonds, the. Atoms will covalently bond with other atoms in order to gain. Covalent Model Of Bonding.
From overallscience.com
Covalent bond (covalency) and its type Overall Science Covalent Model Of Bonding This model originated with the theory developed by g.n. The shared pair of electrons provides each. Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. Covalent bonding occurs when pairs of electrons are shared by atoms. Covalent bonds are formed between two atoms when both have. Covalent Model Of Bonding.
From pediaa.com
How are Covalent Bonds Formed Concept of Chemical Bonds, Basis of Covalent Model Of Bonding The shared pair of electrons provides each. Two models have been developed to describe covalent bonding: Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. This. Covalent Model Of Bonding.
From www.youtube.com
Types of Bonding (Ionic, Covalent, Metallic) GCSE Chemistry Revision Covalent Model Of Bonding Each model has its strengths and weaknesses, and chemists. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. Covalent bonding occurs when pairs of electrons are shared by atoms. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both. Covalent Model Of Bonding.
From joisqugyk.blob.core.windows.net
Models Of Covalent Bonding at Alyce Smith blog Covalent Model Of Bonding This model originated with the theory developed by g.n. The diatomic hydrogen molecule (h 2) is the simplest model of a covalent bond, and is represented in lewis structures as: Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Covalent bonds are formed between two atoms when both have similar tendencies. Covalent Model Of Bonding.
From www.science-revision.co.uk
Covalent bonding Covalent Model Of Bonding This model originated with the theory developed by g.n. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. In the simplest model (figure 1a) a covalent bond is represented by a straight line between the atoms shown by their element symbols. The shared pair of electrons provides each.. Covalent Model Of Bonding.
From joisqugyk.blob.core.windows.net
Models Of Covalent Bonding at Alyce Smith blog Covalent Model Of Bonding Each model has its strengths and weaknesses, and chemists. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. Two models have been developed to describe covalent bonding: This. Covalent Model Of Bonding.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Covalent Model Of Bonding The diatomic hydrogen molecule (h 2) is the simplest model of a covalent bond, and is represented in lewis structures as: Valence bond theory and molecular orbital theory. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. The shared pair of electrons provides each. This model originated with the. Covalent Model Of Bonding.
From studymind.co.uk
Covalent Bond Diagrams (GCSE Chemistry) Study Mind Covalent Model Of Bonding Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full. Each model has its strengths and weaknesses, and chemists. Two models have been developed to describe covalent bonding: The. Covalent Model Of Bonding.