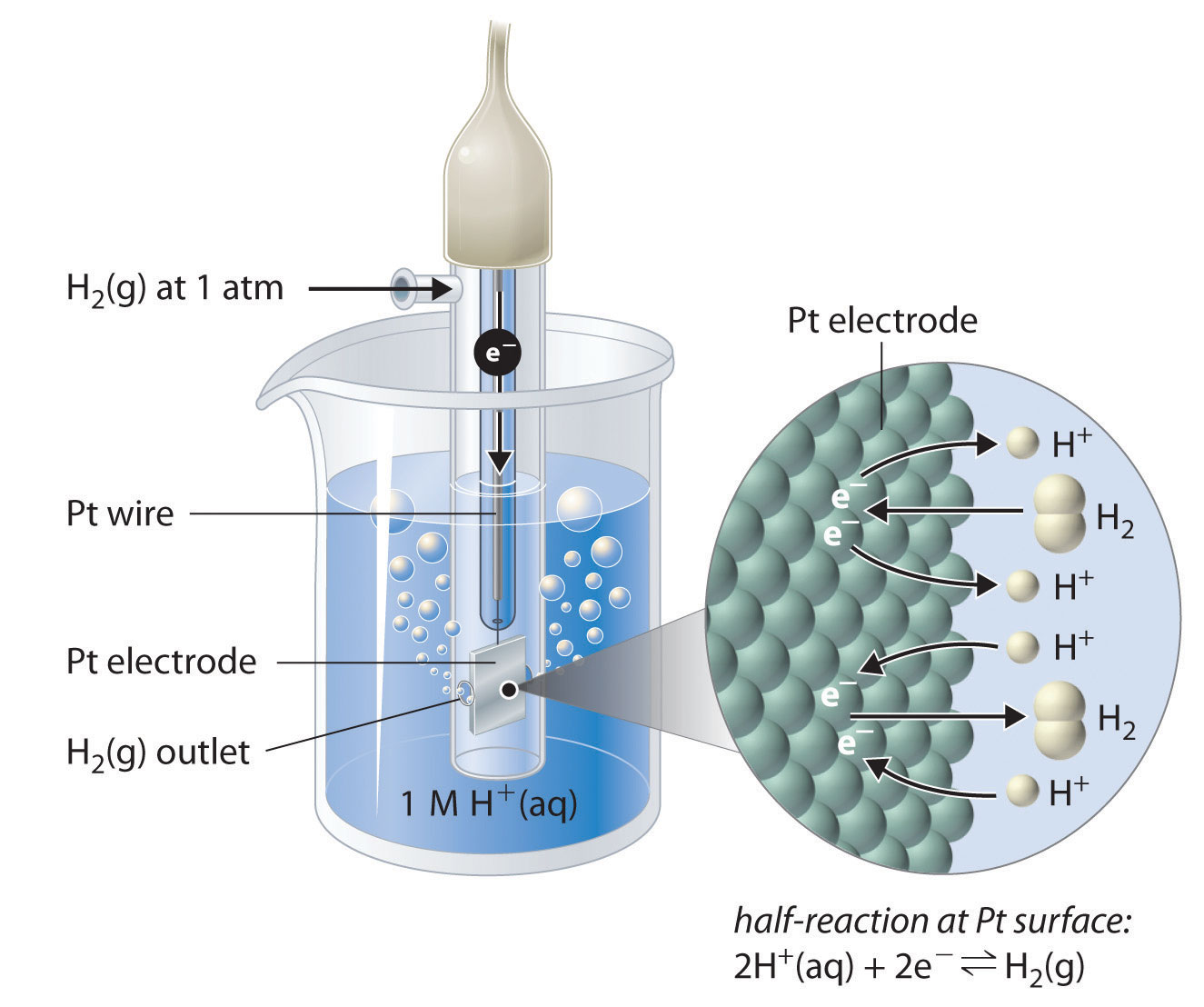
Standard Hydrogen Electrode Half Cell . The value of the standard electrode potential is. The cell potential of an electrochemical cell is the difference between its cathode and anode. The hydrogen electrode consists of. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. If the material conducts electricity, it may be used as an electrode. What is a standard hydrogen. A half cell consists of an electrode and the species to be oxidized or reduced.
from saylordotorg.github.io
What is a standard hydrogen. The cell potential of an electrochemical cell is the difference between its cathode and anode. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The hydrogen electrode consists of. If the material conducts electricity, it may be used as an electrode. A half cell consists of an electrode and the species to be oxidized or reduced. The value of the standard electrode potential is.
Standard Potentials
Standard Hydrogen Electrode Half Cell The cell potential of an electrochemical cell is the difference between its cathode and anode. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The cell potential of an electrochemical cell is the difference between its cathode and anode. If the material conducts electricity, it may be used as an electrode. The hydrogen electrode consists of. A half cell consists of an electrode and the species to be oxidized or reduced. What is a standard hydrogen. The value of the standard electrode potential is.
From www.youtube.com
Standard hydrogen electrode ( SHE ) , EC CELL, NERNST EQUATION BY Standard Hydrogen Electrode Half Cell The value of the standard electrode potential is. A half cell consists of an electrode and the species to be oxidized or reduced. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The cell potential of an electrochemical cell is the difference between its cathode and anode. If the material. Standard Hydrogen Electrode Half Cell.
From www.doubtnut.com
Draw a neat labeled diagram of Standard Hydrogen Electrode (SHE). Write Standard Hydrogen Electrode Half Cell The hydrogen electrode consists of. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. If the material conducts electricity, it may be used as an electrode. What is a standard hydrogen. A half cell consists of an electrode and the species to be oxidized or reduced. The cell potential of. Standard Hydrogen Electrode Half Cell.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Half Cell The cell potential of an electrochemical cell is the difference between its cathode and anode. The hydrogen electrode consists of. The value of the standard electrode potential is. If the material conducts electricity, it may be used as an electrode. What is a standard hydrogen. A half cell consists of an electrode and the species to be oxidized or reduced.. Standard Hydrogen Electrode Half Cell.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Standard Hydrogen Electrode Half Cell The hydrogen electrode consists of. The cell potential of an electrochemical cell is the difference between its cathode and anode. What is a standard hydrogen. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The value of the standard electrode potential is. A half cell consists of an electrode and. Standard Hydrogen Electrode Half Cell.
From www.numerade.com
SOLVEDFour voltaic cells are set up. In each, one halfcell contains a Standard Hydrogen Electrode Half Cell The cell potential of an electrochemical cell is the difference between its cathode and anode. The hydrogen electrode consists of. The value of the standard electrode potential is. A half cell consists of an electrode and the species to be oxidized or reduced. What is a standard hydrogen. If the material conducts electricity, it may be used as an electrode.. Standard Hydrogen Electrode Half Cell.
From question.pandai.org
Standard Electrode Potential Standard Hydrogen Electrode Half Cell If the material conducts electricity, it may be used as an electrode. The value of the standard electrode potential is. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The cell potential of an electrochemical cell is the difference between its cathode and anode. The hydrogen electrode consists of. What. Standard Hydrogen Electrode Half Cell.
From wisc.pb.unizin.org
D40.3 Standard HalfCell Potentials Chemistry 109 Fall 2021 Standard Hydrogen Electrode Half Cell The cell potential of an electrochemical cell is the difference between its cathode and anode. What is a standard hydrogen. If the material conducts electricity, it may be used as an electrode. A half cell consists of an electrode and the species to be oxidized or reduced. The value of the standard electrode potential is. Standard hydrogen electrode is used. Standard Hydrogen Electrode Half Cell.
From cider.uoregon.edu
Standard Hydrogen Electrode Demonstration and AACT Simulation CIDER Standard Hydrogen Electrode Half Cell A half cell consists of an electrode and the species to be oxidized or reduced. What is a standard hydrogen. The cell potential of an electrochemical cell is the difference between its cathode and anode. If the material conducts electricity, it may be used as an electrode. The hydrogen electrode consists of. The value of the standard electrode potential is.. Standard Hydrogen Electrode Half Cell.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Standard Hydrogen Electrode Half Cell Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. A half cell consists of an electrode and the species to be oxidized or reduced. The value of the standard electrode potential is. The hydrogen electrode consists of. What is a standard hydrogen. If the material conducts electricity, it may be. Standard Hydrogen Electrode Half Cell.
From scienceinfo.com
What is Standard Hydrogen Electrode? Standard Hydrogen Electrode Half Cell The hydrogen electrode consists of. The cell potential of an electrochemical cell is the difference between its cathode and anode. What is a standard hydrogen. If the material conducts electricity, it may be used as an electrode. The value of the standard electrode potential is. A half cell consists of an electrode and the species to be oxidized or reduced.. Standard Hydrogen Electrode Half Cell.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Half Cell The cell potential of an electrochemical cell is the difference between its cathode and anode. The hydrogen electrode consists of. If the material conducts electricity, it may be used as an electrode. A half cell consists of an electrode and the species to be oxidized or reduced. The value of the standard electrode potential is. What is a standard hydrogen.. Standard Hydrogen Electrode Half Cell.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Half Cell A half cell consists of an electrode and the species to be oxidized or reduced. The value of the standard electrode potential is. The hydrogen electrode consists of. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The cell potential of an electrochemical cell is the difference between its cathode. Standard Hydrogen Electrode Half Cell.
From www.dreamstime.com
A Standard Hydrogen Electrode (she) is an Electrode that Scientists Use Standard Hydrogen Electrode Half Cell What is a standard hydrogen. The hydrogen electrode consists of. The value of the standard electrode potential is. A half cell consists of an electrode and the species to be oxidized or reduced. The cell potential of an electrochemical cell is the difference between its cathode and anode. If the material conducts electricity, it may be used as an electrode.. Standard Hydrogen Electrode Half Cell.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Standard Hydrogen Electrode Half Cell The cell potential of an electrochemical cell is the difference between its cathode and anode. The value of the standard electrode potential is. If the material conducts electricity, it may be used as an electrode. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The hydrogen electrode consists of. What. Standard Hydrogen Electrode Half Cell.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Half Cell What is a standard hydrogen. The value of the standard electrode potential is. A half cell consists of an electrode and the species to be oxidized or reduced. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The hydrogen electrode consists of. The cell potential of an electrochemical cell is. Standard Hydrogen Electrode Half Cell.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Half Cell The value of the standard electrode potential is. If the material conducts electricity, it may be used as an electrode. What is a standard hydrogen. The cell potential of an electrochemical cell is the difference between its cathode and anode. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. A. Standard Hydrogen Electrode Half Cell.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID4072119 Standard Hydrogen Electrode Half Cell If the material conducts electricity, it may be used as an electrode. What is a standard hydrogen. A half cell consists of an electrode and the species to be oxidized or reduced. The cell potential of an electrochemical cell is the difference between its cathode and anode. Standard hydrogen electrode is used as a reference electrode when calculating the standard. Standard Hydrogen Electrode Half Cell.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Half Cell Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The hydrogen electrode consists of. What is a standard hydrogen. The cell potential of an electrochemical cell is the difference between its cathode and anode. The value of the standard electrode potential is. If the material conducts electricity, it may be. Standard Hydrogen Electrode Half Cell.
From saylordotorg.github.io
Standard Potentials Standard Hydrogen Electrode Half Cell The hydrogen electrode consists of. What is a standard hydrogen. If the material conducts electricity, it may be used as an electrode. The cell potential of an electrochemical cell is the difference between its cathode and anode. A half cell consists of an electrode and the species to be oxidized or reduced. Standard hydrogen electrode is used as a reference. Standard Hydrogen Electrode Half Cell.
From byjus.com
What is standard hydrogen electrode? Standard Hydrogen Electrode Half Cell What is a standard hydrogen. A half cell consists of an electrode and the species to be oxidized or reduced. The cell potential of an electrochemical cell is the difference between its cathode and anode. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The value of the standard electrode. Standard Hydrogen Electrode Half Cell.
From www.savemyexams.com
The Hydrogen Electrode (HL) HL IB Chemistry Revision Notes 2025 Standard Hydrogen Electrode Half Cell If the material conducts electricity, it may be used as an electrode. A half cell consists of an electrode and the species to be oxidized or reduced. The hydrogen electrode consists of. The value of the standard electrode potential is. The cell potential of an electrochemical cell is the difference between its cathode and anode. Standard hydrogen electrode is used. Standard Hydrogen Electrode Half Cell.
From www.slideserve.com
PPT Chemistry 100 Chapter 20 PowerPoint Presentation, free download Standard Hydrogen Electrode Half Cell Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. A half cell consists of an electrode and the species to be oxidized or reduced. If the material conducts electricity, it may be used as an electrode. The hydrogen electrode consists of. The value of the standard electrode potential is. What. Standard Hydrogen Electrode Half Cell.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Standard Hydrogen Electrode Half Cell What is a standard hydrogen. The cell potential of an electrochemical cell is the difference between its cathode and anode. The value of the standard electrode potential is. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. If the material conducts electricity, it may be used as an electrode. A. Standard Hydrogen Electrode Half Cell.
From www.hanlin.com
CIE A Level Chemistry复习笔记5.3.4 Measuring the Standard Electrode Standard Hydrogen Electrode Half Cell Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The cell potential of an electrochemical cell is the difference between its cathode and anode. What is a standard hydrogen. If the material conducts electricity, it may be used as an electrode. A half cell consists of an electrode and the. Standard Hydrogen Electrode Half Cell.
From www.slideserve.com
PPT Electroanalysis PowerPoint Presentation, free download ID6733376 Standard Hydrogen Electrode Half Cell If the material conducts electricity, it may be used as an electrode. The hydrogen electrode consists of. A half cell consists of an electrode and the species to be oxidized or reduced. The value of the standard electrode potential is. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. What. Standard Hydrogen Electrode Half Cell.
From sulekharanirxiichemistry.blogspot.com
SULEKHA Class XII Chemistry STANDARD HYDROGEN ELECTRODE Standard Hydrogen Electrode Half Cell What is a standard hydrogen. A half cell consists of an electrode and the species to be oxidized or reduced. The hydrogen electrode consists of. If the material conducts electricity, it may be used as an electrode. The cell potential of an electrochemical cell is the difference between its cathode and anode. Standard hydrogen electrode is used as a reference. Standard Hydrogen Electrode Half Cell.
From brainly.in
What is standard hydrogen electrode? How is it prepared? Explain with Standard Hydrogen Electrode Half Cell If the material conducts electricity, it may be used as an electrode. What is a standard hydrogen. The hydrogen electrode consists of. The value of the standard electrode potential is. A half cell consists of an electrode and the species to be oxidized or reduced. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential. Standard Hydrogen Electrode Half Cell.
From monomole.com
Standard hydrogen electrode Mono Mole Standard Hydrogen Electrode Half Cell Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The cell potential of an electrochemical cell is the difference between its cathode and anode. The hydrogen electrode consists of. If the material conducts electricity, it may be used as an electrode. What is a standard hydrogen. A half cell consists. Standard Hydrogen Electrode Half Cell.
From gaskatel.de
Principles of the pHvalue measurement with hydrogen electrodes Gaskatel Standard Hydrogen Electrode Half Cell The value of the standard electrode potential is. A half cell consists of an electrode and the species to be oxidized or reduced. What is a standard hydrogen. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The hydrogen electrode consists of. The cell potential of an electrochemical cell is. Standard Hydrogen Electrode Half Cell.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Half Cell If the material conducts electricity, it may be used as an electrode. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The cell potential of an electrochemical cell is the difference between its cathode and anode. The value of the standard electrode potential is. The hydrogen electrode consists of. A. Standard Hydrogen Electrode Half Cell.
From mmerevise.co.uk
Electrochemical Cells MME Standard Hydrogen Electrode Half Cell The hydrogen electrode consists of. The value of the standard electrode potential is. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. A half cell consists of an electrode and the species to be oxidized or reduced. The cell potential of an electrochemical cell is the difference between its cathode. Standard Hydrogen Electrode Half Cell.
From www.youtube.com
Half cells and the standard hydrogen electrode YouTube Standard Hydrogen Electrode Half Cell The value of the standard electrode potential is. A half cell consists of an electrode and the species to be oxidized or reduced. The hydrogen electrode consists of. What is a standard hydrogen. If the material conducts electricity, it may be used as an electrode. The cell potential of an electrochemical cell is the difference between its cathode and anode.. Standard Hydrogen Electrode Half Cell.
From revise.im
Redox and Half Cells Revise.im Standard Hydrogen Electrode Half Cell A half cell consists of an electrode and the species to be oxidized or reduced. The cell potential of an electrochemical cell is the difference between its cathode and anode. The hydrogen electrode consists of. If the material conducts electricity, it may be used as an electrode. The value of the standard electrode potential is. What is a standard hydrogen.. Standard Hydrogen Electrode Half Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4491325 Standard Hydrogen Electrode Half Cell A half cell consists of an electrode and the species to be oxidized or reduced. The value of the standard electrode potential is. The hydrogen electrode consists of. If the material conducts electricity, it may be used as an electrode. The cell potential of an electrochemical cell is the difference between its cathode and anode. What is a standard hydrogen.. Standard Hydrogen Electrode Half Cell.
From cider.uoregon.edu
Standard Hydrogen Electrode Demonstration and AACT Simulation CIDER Standard Hydrogen Electrode Half Cell The cell potential of an electrochemical cell is the difference between its cathode and anode. What is a standard hydrogen. The value of the standard electrode potential is. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The hydrogen electrode consists of. A half cell consists of an electrode and. Standard Hydrogen Electrode Half Cell.