Iron Iii Oxide Reacts With Hydrochloric Acid . The balanced equation for this reaction is: Enter a chemical equation and get a balanced equation and the type of reaction. When iron(iii) oxide reacts with hydrochloric acid, iron(iii) chloride and water are formed. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. From the balanced equation, we can see that 1 mole of iron (iii) oxide reacts with 6 moles of hydrochloric acid to produce 2 moles. Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g) is. By stoichiometry of the reaction: The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows: How many grams of iron(iii) chloride are formed from 10.0. Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. Use uppercase for the first character in the element and. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced.
from www.chegg.com
When iron(iii) oxide reacts with hydrochloric acid, iron(iii) chloride and water are formed. From the balanced equation, we can see that 1 mole of iron (iii) oxide reacts with 6 moles of hydrochloric acid to produce 2 moles. When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced. How many grams of iron(iii) chloride are formed from 10.0. By stoichiometry of the reaction: Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows: Use uppercase for the first character in the element and. Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g) is. The balanced equation for this reaction is:
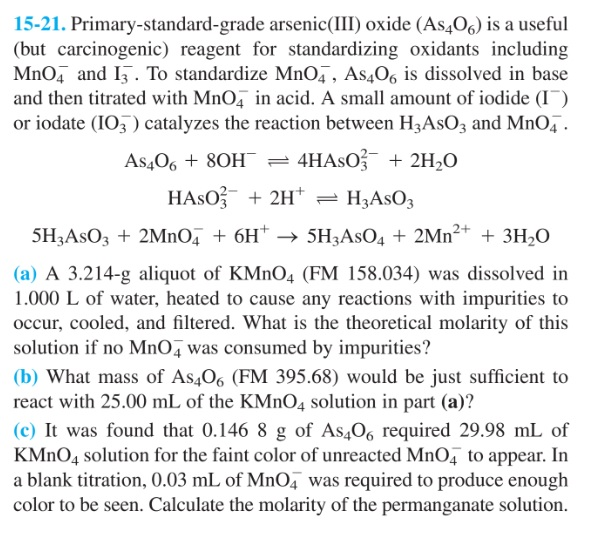
Solved 1521. Primarystandardgrade arsenic(III) oxide
Iron Iii Oxide Reacts With Hydrochloric Acid How many grams of iron(iii) chloride are formed from 10.0. By stoichiometry of the reaction: Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. From the balanced equation, we can see that 1 mole of iron (iii) oxide reacts with 6 moles of hydrochloric acid to produce 2 moles. Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g) is. When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced. The balanced equation for this reaction is: When iron(iii) oxide reacts with hydrochloric acid, iron(iii) chloride and water are formed. Use uppercase for the first character in the element and. Enter a chemical equation and get a balanced equation and the type of reaction. How many grams of iron(iii) chloride are formed from 10.0. The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows:
From www.numerade.com
SOLVED Solid iron (Il) sulfide reacts with hydrochloric acid to form Iron Iii Oxide Reacts With Hydrochloric Acid Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. When iron(iii) oxide reacts with hydrochloric acid, iron(iii) chloride and water are formed. When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced. How many grams of iron(iii) chloride are formed. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.coursehero.com
[Solved] . 6. Iron (I] oxide reacts with HCI acid to produce iron Iron Iii Oxide Reacts With Hydrochloric Acid The balanced equation for this reaction is: The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows: Enter a chemical equation and get a balanced equation and the type of reaction. Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g) is. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED For the following reaction, 4.09 grams of hydrochloric acid are Iron Iii Oxide Reacts With Hydrochloric Acid Use uppercase for the first character in the element and. Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g) is.. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid Iron Iii Oxide Reacts With Hydrochloric Acid The balanced equation for this reaction is: Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. How many grams of iron(iii) chloride are formed from 10.0. Enter a chemical equation and get a balanced equation and the type of reaction. Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED The thermite reaction involves aluminum and iron(III) oxide Iron Iii Oxide Reacts With Hydrochloric Acid By stoichiometry of the reaction: When iron(iii) oxide reacts with hydrochloric acid, iron(iii) chloride and water are formed. The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows: Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Text Writing and Balancing Chemical Equations Write balanced Iron Iii Oxide Reacts With Hydrochloric Acid Use uppercase for the first character in the element and. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g) is. By stoichiometry of the reaction: How many grams of iron(iii) chloride are formed from 10.0. The balanced equation for this. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.youtube.com
Thermit reaction iron (III) oxide reacts with aluminium and gives Iron Iii Oxide Reacts With Hydrochloric Acid The balanced equation for this reaction is: When iron(iii) oxide reacts with hydrochloric acid, iron(iii) chloride and water are formed. Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g) is. The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows: Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. When hydrochloric. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.coursehero.com
[Solved] 14) Iron (III) oxide reacts with carbon monoxide to produce Iron Iii Oxide Reacts With Hydrochloric Acid Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. How many grams of iron(iii) chloride are formed from 10.0. By stoichiometry of the reaction: From the balanced equation, we can see that 1 mole of iron (iii) oxide reacts with 6 moles of hydrochloric acid to produce 2 moles. Enter an ionic equation and get its complete. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.chegg.com
Solved 1521. Primarystandardgrade arsenic(III) oxide Iron Iii Oxide Reacts With Hydrochloric Acid When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced. The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows: Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Enter a chemical equation and get a balanced equation and the type of reaction. The. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Text Writing and Balancing Chemical Equations Write balanced Iron Iii Oxide Reacts With Hydrochloric Acid How many grams of iron(iii) chloride are formed from 10.0. When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced. By stoichiometry of the reaction: From the balanced equation, we can see that 1 mole of iron (iii) oxide reacts with 6 moles of hydrochloric acid to produce 2 moles. The chemical equation for the reaction of. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Iron reacts with oxygen to form iron(III) oxide according to Iron Iii Oxide Reacts With Hydrochloric Acid Enter a chemical equation and get a balanced equation and the type of reaction. Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. By stoichiometry of the reaction: When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced. The balanced equation for this reaction is: Enter an ionic equation and get its complete and. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.sciencephoto.com
Iron (III) oxide in water and in hydrochloric acid Stock Image C036 Iron Iii Oxide Reacts With Hydrochloric Acid Enter a chemical equation and get a balanced equation and the type of reaction. How many grams of iron(iii) chloride are formed from 10.0. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. By stoichiometry of the reaction: Iron reacts with hydrochloric acid. Iron Iii Oxide Reacts With Hydrochloric Acid.
From hxefmjwkk.blob.core.windows.net
Iron Iii Oxide Acid Or Base at Victor Usher blog Iron Iii Oxide Reacts With Hydrochloric Acid When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. From the balanced equation, we can see that 1 mole of iron (iii) oxide reacts with 6 moles of hydrochloric acid to produce 2 moles. Use uppercase for the. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED [References] Use the References to access important values if Iron Iii Oxide Reacts With Hydrochloric Acid How many grams of iron(iii) chloride are formed from 10.0. Enter a chemical equation and get a balanced equation and the type of reaction. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. From the balanced equation, we can see that 1 mole. Iron Iii Oxide Reacts With Hydrochloric Acid.
From stock.adobe.com
Vetor de Redox Reaction Infographic Diagram with example of manganese Iron Iii Oxide Reacts With Hydrochloric Acid From the balanced equation, we can see that 1 mole of iron (iii) oxide reacts with 6 moles of hydrochloric acid to produce 2 moles. The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows: Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. Use uppercase for the first character in the element. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.coursehero.com
[Solved] 14) Iron (III) oxide reacts with carbon monoxide to produce Iron Iii Oxide Reacts With Hydrochloric Acid Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g) is. Enter a chemical equation and get a balanced equation and the type of reaction. Use uppercase for the first character in the element and. When iron(iii) oxide reacts with hydrochloric acid, iron(iii) chloride and water are formed. How many grams of iron(iii) chloride are formed from 10.0. When. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED Iron(III) oxide reacts with carbon monoxide according to the Iron Iii Oxide Reacts With Hydrochloric Acid When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced. By stoichiometry of the reaction: The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows: Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Enter an. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED For the following reaction, 6.84 grams of hydrochloric acid are Iron Iii Oxide Reacts With Hydrochloric Acid The balanced equation for this reaction is: Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. How many grams of iron(iii) chloride are formed from 10.0. Enter a chemical equation and get a balanced equation and the type of reaction. Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g). Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.coursehero.com
[Solved] Solid iron(III) oxide reacts with hydrogen gas to form solid Iron Iii Oxide Reacts With Hydrochloric Acid When iron(iii) oxide reacts with hydrochloric acid, iron(iii) chloride and water are formed. The balanced equation for this reaction is: By stoichiometry of the reaction: Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g) is. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. From the balanced equation, we. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVED When hydrochloric acid reacts with iron(III) oxide, water and Iron Iii Oxide Reacts With Hydrochloric Acid How many grams of iron(iii) chloride are formed from 10.0. The balanced equation for this reaction is: Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. The chemical equation for the. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.alamy.com
Iron sulphide in hydrochloric acid. Image 4 of 4. Iron sulphide reacts Iron Iii Oxide Reacts With Hydrochloric Acid Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Use uppercase for the first character in the element and. From the balanced equation, we can see that 1 mole of iron (iii) oxide reacts with 6 moles of hydrochloric acid to produce 2. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.chegg.com
Solved Solid iron(III) oxide reacts with carbon to produce Iron Iii Oxide Reacts With Hydrochloric Acid Enter a chemical equation and get a balanced equation and the type of reaction. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. From the balanced equation, we can see that 1 mole of iron (iii) oxide reacts with 6 moles of hydrochloric acid to produce 2 moles. How many grams. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.chegg.com
Solved When iron(III) oxide reacts with hydrochloric acid, Iron Iii Oxide Reacts With Hydrochloric Acid Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g) is. How many grams of iron(iii) chloride are formed from 10.0. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. The chemical equation for the reaction of iron (iii) oxide and hydrochloric. Iron Iii Oxide Reacts With Hydrochloric Acid.
From brainly.in
reaction between titanium oxide and sulphuric acid Brainly.in Iron Iii Oxide Reacts With Hydrochloric Acid By stoichiometry of the reaction: Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. How many grams of iron(iii) chloride are formed from 10.0. Use uppercase for the first character in the element and. When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced. From the balanced equation,. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.numerade.com
SOLVEDConsider the extraction of iron from iron(III) oxide by charcoal Iron Iii Oxide Reacts With Hydrochloric Acid By stoichiometry of the reaction: The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows: Use uppercase for the first character in the element and. When iron(iii) oxide reacts with hydrochloric acid, iron(iii) chloride and water are formed. The balanced equation for this reaction is: When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.nagwa.com
Question Video Identifying the Products When a Metal Oxide Reacts with Iron Iii Oxide Reacts With Hydrochloric Acid Use uppercase for the first character in the element and. By stoichiometry of the reaction: Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. The balanced equation for this reaction is: How many grams of iron(iii) chloride are formed from 10.0. From the. Iron Iii Oxide Reacts With Hydrochloric Acid.
From pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry Iron Iii Oxide Reacts With Hydrochloric Acid When iron(iii) oxide reacts with hydrochloric acid, iron(iii) chloride and water are formed. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g) is. The balanced equation for this reaction is: By stoichiometry. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.vrogue.co
What Is Formed When Calcium Carbonate Reacts With Sul vrogue.co Iron Iii Oxide Reacts With Hydrochloric Acid Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. How many grams of iron(iii) chloride are formed from 10.0. The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows: Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.youtube.com
Balance Fe + HCl = FeCl2 + H2 (Iron and Hydrochloric Acid) YouTube Iron Iii Oxide Reacts With Hydrochloric Acid Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced. Enter a chemical equation and get a balanced equation and the type of reaction. From the balanced equation, we can see that 1 mole of iron (iii) oxide reacts with 6 moles of hydrochloric acid. Iron Iii Oxide Reacts With Hydrochloric Acid.
From science-direct-topics.blogspot.com
Science Direct Topics Aluminum Hydrochloric Acid Iron Iii Oxide Reacts With Hydrochloric Acid How many grams of iron(iii) chloride are formed from 10.0. Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows: Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six. Iron Iii Oxide Reacts With Hydrochloric Acid.
From askfilo.com
Recall from Chapter 2, how copper oxide reacts with hydrochloric acid. We.. Iron Iii Oxide Reacts With Hydrochloric Acid When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced. Use uppercase for the first character in the element and. The chemical equation for the reaction of iron (iii) oxide and hydrochloric acid follows: How many grams of iron(iii) chloride are formed from 10.0. By stoichiometry of the reaction: Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.chegg.com
Solved Iron(III) oxide reacts with carbon monoxide according Iron Iii Oxide Reacts With Hydrochloric Acid Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. By stoichiometry of the reaction: From the balanced equation, we can see that 1 mole of iron (iii) oxide reacts with 6. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.sciencephoto.com
Iron (III) oxide in hydrochloric acid Stock Image C036/3132 Iron Iii Oxide Reacts With Hydrochloric Acid Fe(s) + 2hcl(aq) => fecl_2(aq) + h_2(g) what mass of h_2(g) is. How many grams of iron(iii) chloride are formed from 10.0. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. By stoichiometry of the reaction: The chemical equation for the reaction of. Iron Iii Oxide Reacts With Hydrochloric Acid.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) Redox Iron Iii Oxide Reacts With Hydrochloric Acid When hydrochloric acid reacts with iron(iii) oxide, water and iron(iii) chloride are produced. Iron reacts with hydrochloric acid to produce iron(ii) chloride and hydrogen gas. Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. By stoichiometry of the reaction: Fe(s) + 2hcl(aq) =>. Iron Iii Oxide Reacts With Hydrochloric Acid.
From hxefmjwkk.blob.core.windows.net
Iron Iii Oxide Acid Or Base at Victor Usher blog Iron Iii Oxide Reacts With Hydrochloric Acid Fe2o3 + hcl = fecl3 + h2o is a double displacement (metathesis) reaction where one mole of hematite [fe 2 o 3] and six moles of. The balanced equation for this reaction is: How many grams of iron(iii) chloride are formed from 10.0. From the balanced equation, we can see that 1 mole of iron (iii) oxide reacts with 6. Iron Iii Oxide Reacts With Hydrochloric Acid.