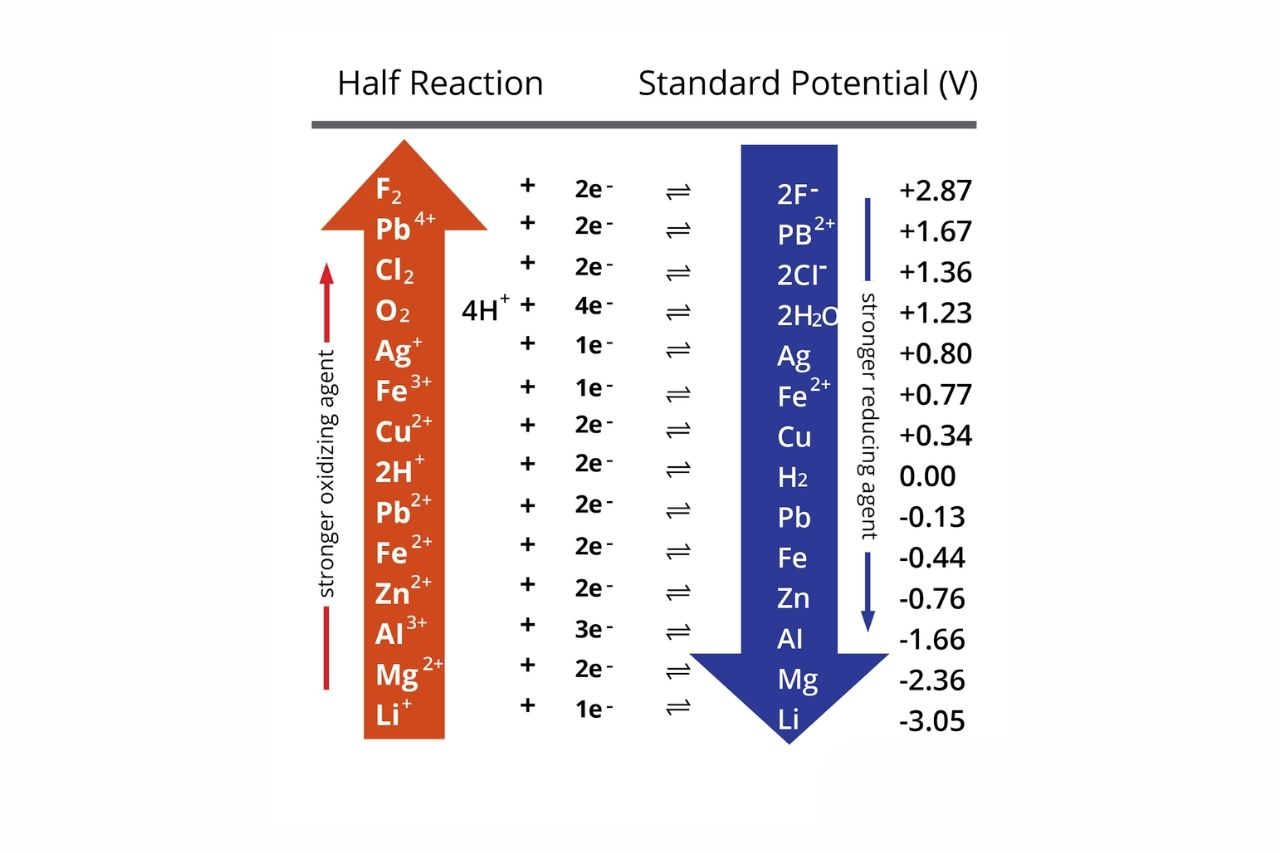
What Is Electrochemical Reactivity . The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Because the potential of these cells to do work by. Electrochemistry is the study of chemical processes that cause electrons to move.
from facts.net
Because the potential of these cells to do work by. Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into.
19 Mindblowing Facts About Electrochemical Series
What Is Electrochemical Reactivity Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Because the potential of these cells to do work by. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. Electrochemistry is the study of chemical processes that cause electrons to move.
From www.msjchem.com
Reactivity 3.2 Electron transfer reactions MSJChem Tutorial videos What Is Electrochemical Reactivity Because the potential of these cells to do work by. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Electrochemistry is the study of chemical processes that cause electrons to move. Metallurgy. What Is Electrochemical Reactivity.
From selftution.com
Reactivity Series of Metals and Nonmetals » Selftution What Is Electrochemical Reactivity The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. Because the potential of these cells to do work by. Electrochemistry is the study of chemical processes. What Is Electrochemical Reactivity.
From classnotes.org.in
Electrochemical Series Chemistry, Class 12, Electro Chemistry What Is Electrochemical Reactivity The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Electrochemistry is the study of chemical processes that cause electrons to move. Because the potential of these cells to do work by. An. What Is Electrochemical Reactivity.
From www.youtube.com
What Is The Electrochemical Series Reactions Chemistry FuseSchool What Is Electrochemical Reactivity The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Electrochemistry is the study of chemical processes that cause electrons to move. Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. An electrochemical cell is any device that converts chemical energy into. What Is Electrochemical Reactivity.
From www.slideshare.net
IB Chemistry on Reactivity Series vs Electrochemical Series What Is Electrochemical Reactivity Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical reactions entail the transfer of electrons to or from a. What Is Electrochemical Reactivity.
From revisechemistry.uk
Reactivity of Metals AQA C4 revisechemistry.uk What Is Electrochemical Reactivity Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemistry is the study of chemical. What Is Electrochemical Reactivity.
From www.slideshare.net
Form 4 Chapter 6 Chemistry Electrochemical Series What Is Electrochemical Reactivity An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or. What Is Electrochemical Reactivity.
From www.vrogue.co
What Is An Easy Way To Remember The Metal Reactivity vrogue.co What Is Electrochemical Reactivity Because the potential of these cells to do work by. Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Electrochemical reactions entail the. What Is Electrochemical Reactivity.
From mrcartlidgesaigon.edublogs.org
10.2 Reactivity Series Mr Cartlidge's second Science Blog What Is Electrochemical Reactivity Because the potential of these cells to do work by. Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical. What Is Electrochemical Reactivity.
From mungfali.com
What Is Electrochemical Series What Is Electrochemical Reactivity An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. Because the potential of these cells to do. What Is Electrochemical Reactivity.
From www.semanticscholar.org
Figure 2 from Reactivity series, activity series and electrochemical What Is Electrochemical Reactivity Electrochemistry is the study of chemical processes that cause electrons to move. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemical reactions entail the transfer of electrons to or from a. What Is Electrochemical Reactivity.
From www.slideshare.net
6.3 (a) electrolysis of an aqueous solution What Is Electrochemical Reactivity The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Electrochemistry is the study of chemical processes that cause electrons to move. Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. An electrochemical cell is any device that converts chemical energy into. What Is Electrochemical Reactivity.
From www.quelpr.com
CSEC Chemistry Reactivity of Metals What Is Electrochemical Reactivity Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Electrochemistry is the study of chemical processes that cause electrons to move. Metallurgy and the extraction of. What Is Electrochemical Reactivity.
From brokeasshome.com
Periodic Table Reactivity Series What Is Electrochemical Reactivity The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Because the potential of these cells to do work by. Metallurgy and the extraction of metals from ores depend on the reactivity and. What Is Electrochemical Reactivity.
From www.vrogue.co
What Is Electrochemical Series vrogue.co What Is Electrochemical Reactivity Electrochemistry is the study of chemical processes that cause electrons to move. Because the potential of these cells to do work by. Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical. What Is Electrochemical Reactivity.
From www.semanticscholar.org
[PDF] Reactivity series, activity series and electrochemical series What Is Electrochemical Reactivity Electrochemistry is the study of chemical processes that cause electrons to move. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Metallurgy and the extraction of metals from ores depend on the. What Is Electrochemical Reactivity.
From www.worksheetsplanet.com
What is Reactivity Definition of Reactivity What Is Electrochemical Reactivity An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Electrochemistry is the study of chemical processes that cause electrons to move. Because the potential of these cells to do work by. The electrochemical series is. What Is Electrochemical Reactivity.
From facts.net
19 Mindblowing Facts About Electrochemical Series What Is Electrochemical Reactivity The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Because the potential of these cells to do work by. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical. What Is Electrochemical Reactivity.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is Electrochemical Reactivity Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Because the potential of these cells to do work by. An electrochemical cell is any device that converts chemical energy into electrical energy,. What Is Electrochemical Reactivity.
From www.thoughtco.com
Electrochemical Cell Definition What Is Electrochemical Reactivity Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Electrochemistry is the study of chemical processes that cause electrons to move. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Because the potential of these cells to do work by. Electrochemical. What Is Electrochemical Reactivity.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu What Is Electrochemical Reactivity Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Electrochemistry is the study of chemical processes that cause electrons to move. An electrochemical cell is any. What Is Electrochemical Reactivity.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell What Is Electrochemical Reactivity Because the potential of these cells to do work by. Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Electrochemical reactions entail the transfer of electrons to or from a molecule, atom,. What Is Electrochemical Reactivity.
From www.dynamicscience.com.au
redox reactions electrolysis The difference between electrochemical What Is Electrochemical Reactivity The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the.. What Is Electrochemical Reactivity.
From www.quelpr.com
CSEC Chemistry Preferential Discharge of Ions and the Electrochemical What Is Electrochemical Reactivity An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemistry is the study of chemical processes that cause electrons to move. Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. The electrochemical series is built up by arranging various redox equilibria in order of. What Is Electrochemical Reactivity.
From sciencenotes.org
Activity Series of Metals (Reactivity Series) What Is Electrochemical Reactivity The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Because the potential of these cells to do work by. Electrochemistry is the study of chemical processes that cause electrons to move. Metallurgy. What Is Electrochemical Reactivity.
From leverageedu.com
Reactivity Series Leverage Edu What Is Electrochemical Reactivity Because the potential of these cells to do work by. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemistry is the study of chemical processes that cause electrons to move. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Electrochemical. What Is Electrochemical Reactivity.
From www.askdifference.com
Electrochemical Series vs. Reactivity Series — What’s the Difference? What Is Electrochemical Reactivity Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Electrochemistry is the study of chemical processes that cause electrons to move. The electrochemical series is built up by arranging. What Is Electrochemical Reactivity.
From www.studypage.in
Electrochemical Series Study Page What Is Electrochemical Reactivity The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Because the potential of these cells to do work by. Electrochemistry is the study of chemical processes that cause electrons to move. An. What Is Electrochemical Reactivity.
From svpwiki.com
Sympathetic Vibratory Physics Electromotive Series What Is Electrochemical Reactivity Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Because the potential of these cells to do work by. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Electrochemistry is the study of chemical processes that cause electrons to move. Electrochemical reactions entail the. What Is Electrochemical Reactivity.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is Electrochemical Reactivity Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. Because the potential of these cells to do work by. An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. The electrochemical series is built up by arranging various redox. What Is Electrochemical Reactivity.
From 2012books.lardbucket.org
Electrochemistry What Is Electrochemical Reactivity Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Because the potential of these cells to do work by. An electrochemical cell is any device that converts chemical energy into electrical energy,. What Is Electrochemical Reactivity.
From unacademy.com
Electrochemical Series, Features and Importance Unacademy What Is Electrochemical Reactivity The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Electrochemical reactions entail the transfer of. What Is Electrochemical Reactivity.
From www.teachoo.com
Reactivity Series of Metals Chart [and How to remember] Teachoo What Is Electrochemical Reactivity An electrochemical cell is any device that converts chemical energy into electrical energy, or electrical energy into. The electrochemical series is built up by arranging various redox equilibria in order of their standard electrode potentials (redox potentials). Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the.. What Is Electrochemical Reactivity.
From mavink.com
What Is Electrochemical Series What Is Electrochemical Reactivity Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. Because the potential of these cells to do work by. Metallurgy and the extraction of metals from ores depend on the reactivity and electrochemical properties of elements. Electrochemistry is the study of chemical processes that cause electrons. What Is Electrochemical Reactivity.
From www.chemistrylearner.com
Reactivity Series Definition and Chart What Is Electrochemical Reactivity Electrochemical reactions entail the transfer of electrons to or from a molecule, atom, or ion at an interface between an electronic conductor, the. Because the potential of these cells to do work by. Electrochemistry is the study of chemical processes that cause electrons to move. The electrochemical series is built up by arranging various redox equilibria in order of their. What Is Electrochemical Reactivity.