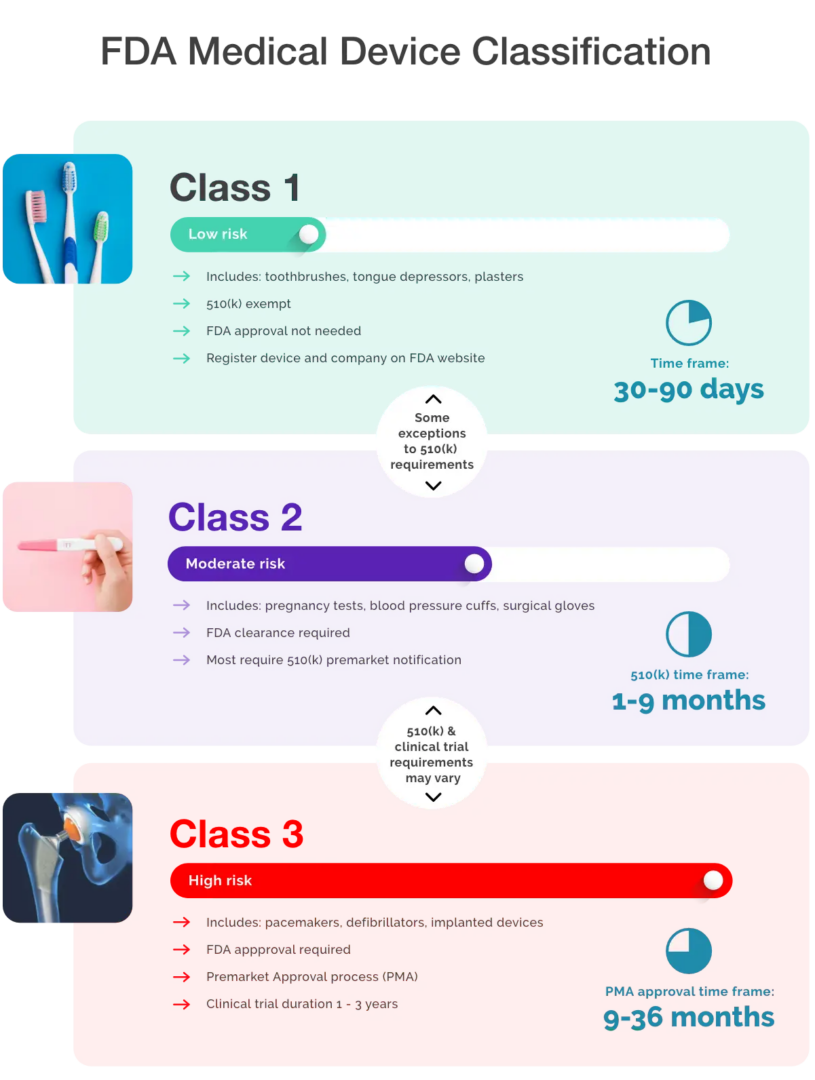
Classification Of Software As A Medical Device . The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software is classified by the european commission’s medical device. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software uses the same risk. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles.
from www.gilero.com
The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software uses the same risk. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software is classified by the european commission’s medical device.
Medical Device Classification Overview of 3 Classes Gilero
Classification Of Software As A Medical Device In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. Medical device software uses the same risk. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software is classified by the european commission’s medical device. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three.
From mavink.com
Medical Device Classification Flowchart Classification Of Software As A Medical Device The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. Medical device software is classified by the european commission’s medical device. Medical device software uses the same risk. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. In. Classification Of Software As A Medical Device.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Classification Of Software As A Medical Device Medical device software is classified by the european commission’s medical device. Medical device software uses the same risk. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. The was. Classification Of Software As A Medical Device.
From www.greenlight.guru
Ultimate Guide to Software as a Medical Device (SaMD) Classification Of Software As A Medical Device The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software is classified by the european commission’s medical device. In addition, the iec 62304 standard for software. Classification Of Software As A Medical Device.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Classification Of Software As A Medical Device Medical device software is classified by the european commission’s medical device. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. The term “software as a medical device” (samd) is defined as software intended to be used for one. Classification Of Software As A Medical Device.
From mavink.com
Fda Medical Device Classification Chart Classification Of Software As A Medical Device In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software uses the same risk. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes. Classification Of Software As A Medical Device.
From medicaldevicehq.com
Different classifications rules for medical device software An Classification Of Software As A Medical Device In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software uses the same risk. Medical device software is classified by the european commission’s medical device. The was. Classification Of Software As A Medical Device.
From www.researchgate.net
Medical Device Classification System Download Table Classification Of Software As A Medical Device Medical device software uses the same risk. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software is classified by the european commission’s medical device. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. The was. Classification Of Software As A Medical Device.
From medicaldevicehq.com
Software Safety Classification Template (IEC 62304, Medical Device Classification Of Software As A Medical Device Medical device software uses the same risk. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software is classified by the european commission’s medical device. In. Classification Of Software As A Medical Device.
From software.boxuang.com
Software Design For Medical Devices Classification Of Software As A Medical Device Medical device software uses the same risk. Medical device software is classified by the european commission’s medical device. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. In. Classification Of Software As A Medical Device.
From www.presentationeze.com
FDA medical device classification PresentationEZE Classification Of Software As A Medical Device Medical device software is classified by the european commission’s medical device. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. The was implemented in 2010 to provide a pathway. Classification Of Software As A Medical Device.
From www.sycaimedical.com
Overview on the regulatory path for software medical devices Classification Of Software As A Medical Device In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software uses the same risk. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. Medical device software is classified by the european commission’s medical device. The term “software as a medical device” (samd) is defined as. Classification Of Software As A Medical Device.
From mungfali.com
Classification Of Medical Devices Classification Of Software As A Medical Device The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software is. Classification Of Software As A Medical Device.
From medicaldevicehq.com
An overview of the IEC 62304 standard and software safety Classification Of Software As A Medical Device In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. Medical device software uses the same risk. Medical device software is classified by the european commission’s medical device. The term “software as a medical device” (samd) is defined as. Classification Of Software As A Medical Device.
From www.complianceandrisks.com
Software as a Medical Device inar Compliance and Risks Classification Of Software As A Medical Device The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. Medical device software is classified by the european commission’s medical device. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software uses the same risk. In. Classification Of Software As A Medical Device.
From www.greenlight.guru
Medical Device Classifications Determine Your Device Class Classification Of Software As A Medical Device Medical device software uses the same risk. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software is classified by the european commission’s medical device. The was. Classification Of Software As A Medical Device.
From operonstrategist.com
Software as a Medical Device (SaMD) IEC 62304 Certification Classification Of Software As A Medical Device Medical device software is classified by the european commission’s medical device. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software uses the same risk. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. In. Classification Of Software As A Medical Device.
From www.youtube.com
Introduction to different classifications rules for medical device Classification Of Software As A Medical Device The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software uses the same risk. Medical device software is classified by the european commission’s medical device. The term “software as a medical device” (samd) is defined as. Classification Of Software As A Medical Device.
From webiomed.ru
New rules for the classification of software as a medical device Classification Of Software As A Medical Device The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software uses the same risk. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. The was implemented in 2010 to provide a pathway for novel devices with. Classification Of Software As A Medical Device.
From kvalito.ch
Classification of Software as a Medical device under Medical Device Classification Of Software As A Medical Device The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software uses the same risk. In addition, the iec 62304 standard for software lifecycle processes of medical. Classification Of Software As A Medical Device.
From www.researchgate.net
Medical Device Classification a Download Scientific Diagram Classification Of Software As A Medical Device The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software is classified by the european commission’s medical device. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software uses the same risk. The was. Classification Of Software As A Medical Device.
From chartexamples.com
Mdr Device Classification Flowchart Chart Examples Classification Of Software As A Medical Device The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. Medical device software is classified by the european commission’s medical device. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software uses the same risk. In. Classification Of Software As A Medical Device.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Classification Of Software As A Medical Device In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. Medical device software is classified by the european commission’s medical device. The term “software as a medical device” (samd) is defined as software intended to be used for one. Classification Of Software As A Medical Device.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Classification Of Software As A Medical Device Medical device software uses the same risk. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software is classified by the european commission’s medical device. In. Classification Of Software As A Medical Device.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Classification Of Software As A Medical Device The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software uses the same risk. The was implemented in 2010 to provide a pathway for novel devices with. Classification Of Software As A Medical Device.
From chinameddevice.com
CFDA New Medical Device Classification Catalog China Med Device Classification Of Software As A Medical Device In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software is classified by the european commission’s medical device. Medical device software uses the same risk. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. The term “software as a medical device” (samd) is defined as. Classification Of Software As A Medical Device.
From mungfali.com
Classification Of Medical Devices Classification Of Software As A Medical Device The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software is classified by the european commission’s medical device. Medical device software uses the same risk. The term “software as a medical device” (samd) is defined as. Classification Of Software As A Medical Device.
From kvalito.ch
Classification of Software as a Medical device under Medical Device Classification Of Software As A Medical Device Medical device software is classified by the european commission’s medical device. Medical device software uses the same risk. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. The was. Classification Of Software As A Medical Device.
From kvalito.ch
What is and what is not a Software as Medical Device (SaMD)? Kvalito Classification Of Software As A Medical Device Medical device software uses the same risk. Medical device software is classified by the european commission’s medical device. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. The term “software as a medical device” (samd) is defined as. Classification Of Software As A Medical Device.
From mdrc-consulting.com
Medical software and gadgets MDRC Classification Of Software As A Medical Device The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software is classified by the european commission’s medical device. Medical device software uses the same risk. The was. Classification Of Software As A Medical Device.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Classification Of Software As A Medical Device Medical device software uses the same risk. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. Medical device software is classified by the european commission’s medical device. In. Classification Of Software As A Medical Device.
From www.jamasoftware.com
FDA Medical Device Class and Classifications Jama Software Classification Of Software As A Medical Device In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software is classified by the european commission’s medical device. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. The was implemented in 2010 to provide a pathway. Classification Of Software As A Medical Device.
From medicaldevicehq.com
Different classifications rules for medical device software An Classification Of Software As A Medical Device The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. Medical device software uses. Classification Of Software As A Medical Device.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Classification Of Software As A Medical Device The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. Medical device software is classified by the european commission’s medical device. In addition, the iec 62304 standard for software. Classification Of Software As A Medical Device.
From kantify.com
Kantify Improving Human Health through Artificial Intelligence Classification Of Software As A Medical Device The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. In addition, the iec 62304 standard for software lifecycle processes of medical devices defines three. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software is. Classification Of Software As A Medical Device.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Classification Of Software As A Medical Device The was implemented in 2010 to provide a pathway for novel devices with lower risk profiles. The term “software as a medical device” (samd) is defined as software intended to be used for one or more medical purposes that perform these. Medical device software is classified by the european commission’s medical device. Medical device software uses the same risk. In. Classification Of Software As A Medical Device.