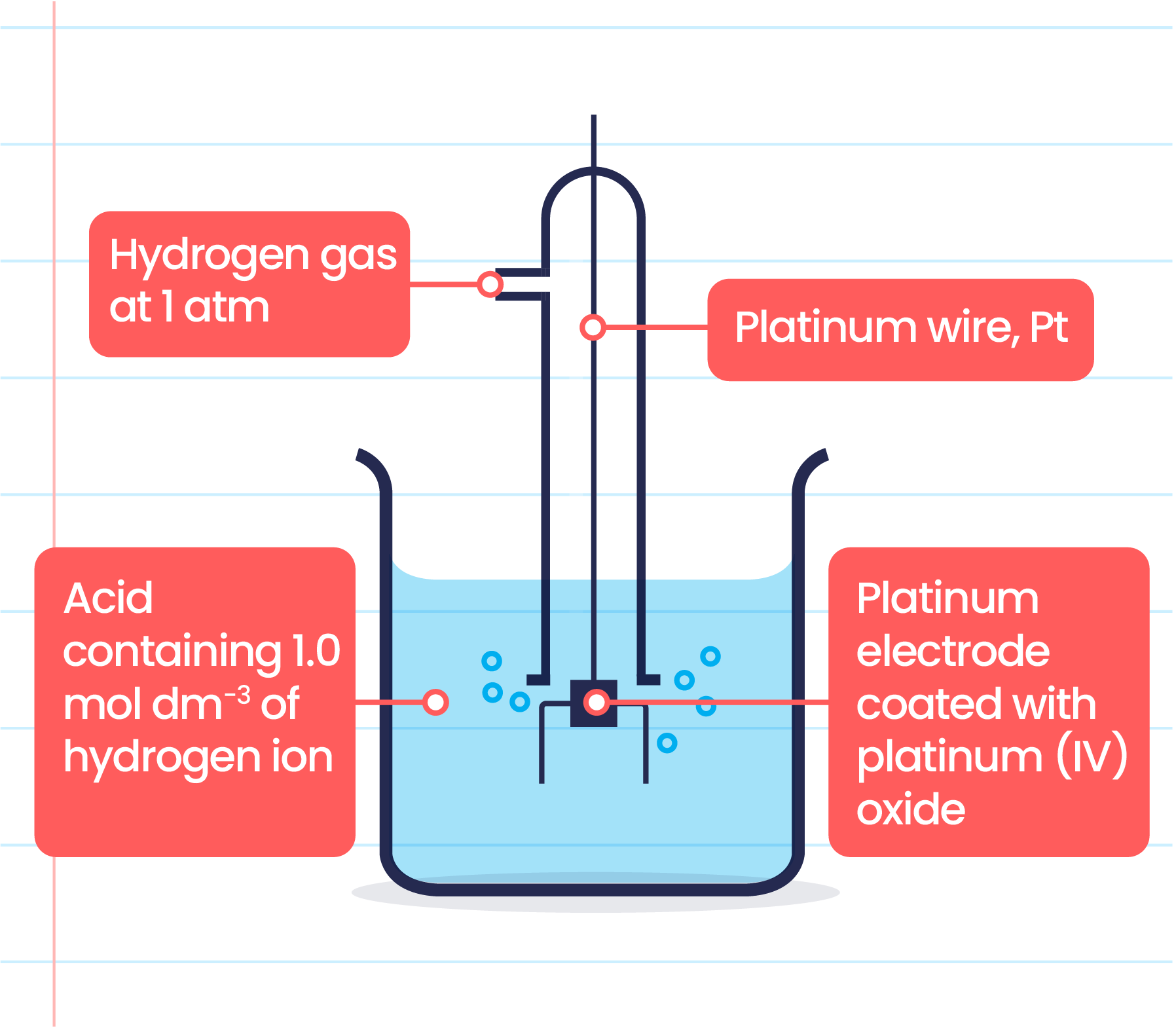
Standard Hydrogen Electrode (She) . This is because it acts as a reference for comparison with any other electrode. We define the electrical potential of this half. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. However, as soon as at least one of these parameters is not unit, the hydrogen reference electrode is the reversible hydrogen electrode (rhe) and its potential deviates from that of the she according to eq 10. H+ +e− → 12h2 h + + e − → 1 2 h 2. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and the hydrogen reference electrode is the standard hydrogen electrode (she). We can then use this system to measure the potentials of. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. We call this e0 e 0, the standard reduction potential.
from app.pandai.org
The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. We call this e0 e 0, the standard reduction potential. H+ +e− → 12h2 h + + e − → 1 2 h 2. In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and the hydrogen reference electrode is the standard hydrogen electrode (she). Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. We can then use this system to measure the potentials of. We define the electrical potential of this half. However, as soon as at least one of these parameters is not unit, the hydrogen reference electrode is the reversible hydrogen electrode (rhe) and its potential deviates from that of the she according to eq 10. This is because it acts as a reference for comparison with any other electrode.
Determine oxidising agent and reducing agent based on value of standard
Standard Hydrogen Electrode (She) We call this e0 e 0, the standard reduction potential. We can then use this system to measure the potentials of. This is because it acts as a reference for comparison with any other electrode. We call this e0 e 0, the standard reduction potential. H+ +e− → 12h2 h + + e − → 1 2 h 2. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and the hydrogen reference electrode is the standard hydrogen electrode (she). We define the electrical potential of this half. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. However, as soon as at least one of these parameters is not unit, the hydrogen reference electrode is the reversible hydrogen electrode (rhe) and its potential deviates from that of the she according to eq 10.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Standard Hydrogen Electrode (She) In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and the hydrogen reference electrode is the standard hydrogen electrode (she). H+ +e− → 12h2 h + + e − → 1 2 h 2. We can then use this system to measure the potentials of. We call this e0 e 0, the. Standard Hydrogen Electrode (She).
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode (She) The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. We call this e0 e 0, the standard reduction potential. In the case of f(h 2) = 1.00. Standard Hydrogen Electrode (She).
From www.chegg.com
Solved A standard hydrogen electrode (SHE) is shown in the Standard Hydrogen Electrode (She) We define the electrical potential of this half. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. H+ +e− → 12h2 h + + e − → 1 2 h 2. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero.. Standard Hydrogen Electrode (She).
From sulekharanirxiichemistry.blogspot.com
SULEKHA Class XII Chemistry STANDARD HYDROGEN ELECTRODE Standard Hydrogen Electrode (She) Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. We can then use this system to measure the potentials of. In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and the hydrogen reference electrode is the standard hydrogen electrode (she). However, as soon as at least one. Standard Hydrogen Electrode (She).
From www.researchgate.net
Absolute and relative (respect to Standard Hydrogen Electrode, SHE Standard Hydrogen Electrode (She) The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. We can then use this system to measure the potentials of. We call this e0 e 0, the standard reduction potential. However, as soon as at least one of these parameters is not unit, the hydrogen reference electrode is the reversible hydrogen. Standard Hydrogen Electrode (She).
From www.youtube.com
class 12//Standard hydrogen electrode (SHE) clear explanation in Telugu Standard Hydrogen Electrode (She) We call this e0 e 0, the standard reduction potential. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. H+ +e− → 12h2 h + + e − → 1 2 h 2. Under these conditions, the potential for the hydrogen reduction is defined as. Standard Hydrogen Electrode (She).
From www.doubtnut.com
Describe the construction and working of the standard hydrogen electr Standard Hydrogen Electrode (She) Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and the hydrogen reference electrode is the standard hydrogen electrode (she). We call this e0 e 0, the standard reduction potential. The standard hydrogen electrode is often abbreviated to. Standard Hydrogen Electrode (She).
From psu.pb.unizin.org
Electrode and Cell Potentials (17.3) Chemistry 110 Standard Hydrogen Electrode (She) In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and the hydrogen reference electrode is the standard hydrogen electrode (she). We can then use this system to measure the potentials of. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. We define the electrical potential of this. Standard Hydrogen Electrode (She).
From askfilo.com
Standard hydrogen electrode (SHE) It is used as reference electrode who.. Standard Hydrogen Electrode (She) We call this e0 e 0, the standard reduction potential. This is because it acts as a reference for comparison with any other electrode. H+ +e− → 12h2 h + + e − → 1 2 h 2. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. However, as soon as at least one of. Standard Hydrogen Electrode (She).
From saylordotorg.github.io
Standard Potentials Standard Hydrogen Electrode (She) We call this e0 e 0, the standard reduction potential. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. We can then use this system to measure. Standard Hydrogen Electrode (She).
From mmerevise.co.uk
Electrochemical Cells MME Standard Hydrogen Electrode (She) Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. We can then use this system to measure the potentials of. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In the case of f(h 2) = 1.00 bar and a(h. Standard Hydrogen Electrode (She).
From www.youtube.com
SHE (Standard Hydrogen Electrode) YouTube Standard Hydrogen Electrode (She) We define the electrical potential of this half. H+ +e− → 12h2 h + + e − → 1 2 h 2. We call this e0 e 0, the standard reduction potential. This is because it acts as a reference for comparison with any other electrode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential. Standard Hydrogen Electrode (She).
From www.youtube.com
The Standard hydrogen electrode (SHE) YouTube Standard Hydrogen Electrode (She) We call this e0 e 0, the standard reduction potential. H+ +e− → 12h2 h + + e − → 1 2 h 2. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. We define the electrical potential of this half. Under these conditions, the potential for the hydrogen reduction is. Standard Hydrogen Electrode (She).
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode (She) Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. We call this e0 e 0, the standard reduction potential. In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and. Standard Hydrogen Electrode (She).
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode (She) H+ +e− → 12h2 h + + e − → 1 2 h 2. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential. Standard Hydrogen Electrode (She).
From www.slideserve.com
PPT Ch. 18 Electrochemistry PowerPoint Presentation, free download Standard Hydrogen Electrode (She) We can then use this system to measure the potentials of. H+ +e− → 12h2 h + + e − → 1 2 h 2. In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and the hydrogen reference electrode is the standard hydrogen electrode (she). We define the electrical potential of this. Standard Hydrogen Electrode (She).
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas Standard Hydrogen Electrode (She) The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. We call this e0 e 0, the standard reduction potential. We can then use this system to measure the potentials of. We define the electrical potential of this half. Under these conditions, the potential for the hydrogen reduction is defined as exactly. Standard Hydrogen Electrode (She).
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode (She) In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and the hydrogen reference electrode is the standard hydrogen electrode (she). This is because it acts as a reference for comparison with any other electrode. We can then use this system to measure the potentials of. The standard hydrogen electrode is the standard. Standard Hydrogen Electrode (She).
From byjus.com
What is standard hydrogen electrode? Standard Hydrogen Electrode (She) The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. We can then use this system to measure the potentials of. Under these conditions, the potential for the. Standard Hydrogen Electrode (She).
From brainly.in
write the symbolic representation of standard hydrogen electrode Standard Hydrogen Electrode (She) We define the electrical potential of this half. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. However, as soon as at least one of these parameters is not unit, the hydrogen reference electrode is the reversible. Standard Hydrogen Electrode (She).
From monomole.com
Standard hydrogen electrode Mono Mole Standard Hydrogen Electrode (She) In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and the hydrogen reference electrode is the standard hydrogen electrode (she). We can then use this system to measure the potentials of. We call this e0 e 0, the standard reduction potential. The standard hydrogen electrode is often abbreviated to she, and its. Standard Hydrogen Electrode (She).
From www.researchgate.net
(PDF) What are Normal Hydrogen Electrode (NHE), Standard Hydrogen Standard Hydrogen Electrode (She) The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. H+ +e− → 12h2 h + + e − → 1 2 h 2. We define the electrical potential of this half.. Standard Hydrogen Electrode (She).
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Standard Hydrogen Electrode (She) We call this e0 e 0, the standard reduction potential. We can then use this system to measure the potentials of. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. We define the electrical potential of this half. This is because it acts as a reference for comparison with any other electrode. In the case. Standard Hydrogen Electrode (She).
From www.slideserve.com
PPT Electroanalysis PowerPoint Presentation, free download ID820788 Standard Hydrogen Electrode (She) Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. H+ +e− → 12h2 h + + e − → 1 2 h 2. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. We call this e0 e 0, the standard reduction potential. We define the electrical. Standard Hydrogen Electrode (She).
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Standard Hydrogen Electrode (She) We call this e0 e 0, the standard reduction potential. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. We can then use this system to measure the potentials of. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In. Standard Hydrogen Electrode (She).
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Standard Hydrogen Electrode (She) In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and the hydrogen reference electrode is the standard hydrogen electrode (she). The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential. Standard Hydrogen Electrode (She).
From www.slideserve.com
PPT CHEM1612 Pharmacy Week 9 Nernst Equation PowerPoint Standard Hydrogen Electrode (She) We define the electrical potential of this half. We can then use this system to measure the potentials of. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. However, as soon as at least one of these parameters is not unit, the hydrogen reference electrode is the reversible hydrogen electrode (rhe) and its potential deviates. Standard Hydrogen Electrode (She).
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode (She) We define the electrical potential of this half. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. We call this e0 e 0, the standard reduction potential. H+ +e− → 12h2 h + + e − → 1 2 h 2. We can then use this system to measure the potentials of. This is because. Standard Hydrogen Electrode (She).
From www.vecteezy.com
A Standard Hydrogen Electrode SHE is an electrode that scientists use Standard Hydrogen Electrode (She) We can then use this system to measure the potentials of. H+ +e− → 12h2 h + + e − → 1 2 h 2. We call this e0 e 0, the standard reduction potential. We define the electrical potential of this half. This is because it acts as a reference for comparison with any other electrode. Under these conditions,. Standard Hydrogen Electrode (She).
From app.pandai.org
Determine oxidising agent and reducing agent based on value of standard Standard Hydrogen Electrode (She) H+ +e− → 12h2 h + + e − → 1 2 h 2. This is because it acts as a reference for comparison with any other electrode. We call this e0 e 0, the standard reduction potential. We define the electrical potential of this half. We can then use this system to measure the potentials of. The standard hydrogen. Standard Hydrogen Electrode (She).
From www.shutterstock.com
Standard Hydrogen Electrode She Electrode That Stock Vector (Royalty Standard Hydrogen Electrode (She) Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. In the case of f(h 2) = 1.00 bar and a(h +) = 1.00, e = e° and the hydrogen reference electrode is the standard hydrogen electrode (she).. Standard Hydrogen Electrode (She).
From www.youtube.com
ElectroChemistry 04 Standard Hydrogen Electrode (SHE) Theory and Standard Hydrogen Electrode (She) We can then use this system to measure the potentials of. We define the electrical potential of this half. This is because it acts as a reference for comparison with any other electrode. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. The standard hydrogen electrode is often abbreviated to she, and its standard electrode. Standard Hydrogen Electrode (She).
From www.researchgate.net
Comparison of the voltage vs. standard hydrogen electrode (SHE Standard Hydrogen Electrode (She) The standard hydrogen electrode is the standard measurement of electrode potential for the thermodynamic scale of redox potentials. Under these conditions, the potential for the hydrogen reduction is defined as exactly zero. We define the electrical potential of this half. However, as soon as at least one of these parameters is not unit, the hydrogen reference electrode is the reversible. Standard Hydrogen Electrode (She).
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode (She) We call this e0 e 0, the standard reduction potential. However, as soon as at least one of these parameters is not unit, the hydrogen reference electrode is the reversible hydrogen electrode (rhe) and its potential deviates from that of the she according to eq 10. We can then use this system to measure the potentials of. Under these conditions,. Standard Hydrogen Electrode (She).
From www.slideserve.com
PPT Introduction to electrochemical techniques PowerPoint Standard Hydrogen Electrode (She) However, as soon as at least one of these parameters is not unit, the hydrogen reference electrode is the reversible hydrogen electrode (rhe) and its potential deviates from that of the she according to eq 10. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k.. Standard Hydrogen Electrode (She).