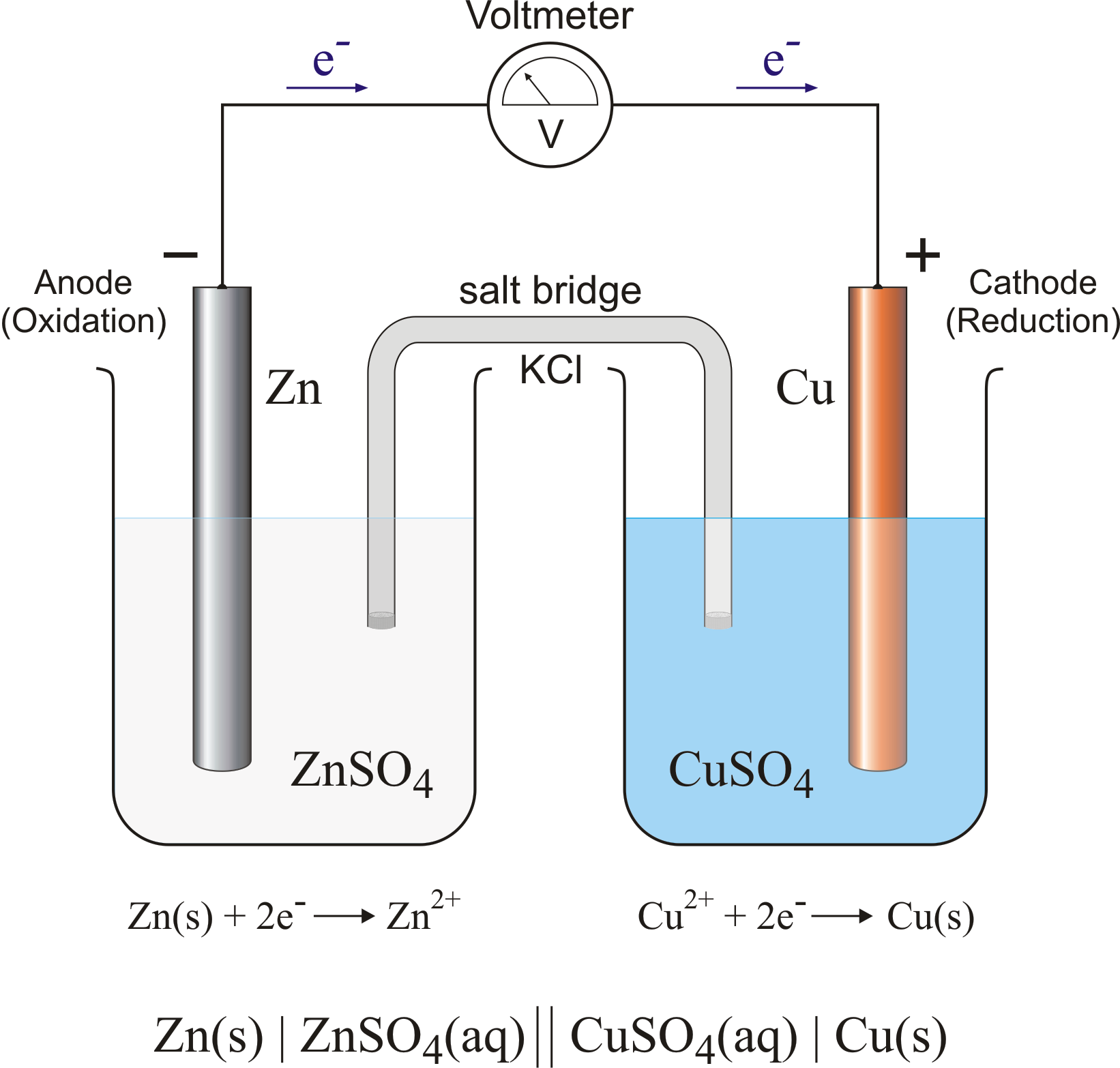
Electrodes In Voltaic Cell . a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is. voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. The structure of a voltaic cell. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. This means that the oxidation will. in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. There are two electrodes in a voltaic cell, one in.
from circuitlibrarylinty.z13.web.core.windows.net
voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is. The structure of a voltaic cell. in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. This means that the oxidation will. There are two electrodes in a voltaic cell, one in.
Voltaic Cell Diagram
Electrodes In Voltaic Cell This means that the oxidation will. There are two electrodes in a voltaic cell, one in. in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is. The structure of a voltaic cell. This means that the oxidation will. voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into.
From wisc.pb.unizin.org
Day 39 Voltaic Cells Chemistry 109 Electrodes In Voltaic Cell a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. This means that the oxidation will. There are two. Electrodes In Voltaic Cell.
From chem2u.blogspot.com
chem2U Simple Voltaic Cell Electrodes In Voltaic Cell The structure of a voltaic cell. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is. There are two electrodes in a voltaic cell, one in. This means that the oxidation will. in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. . Electrodes In Voltaic Cell.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical Electrodes In Voltaic Cell There are two electrodes in a voltaic cell, one in. an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an. Electrodes In Voltaic Cell.
From quizdbpharmacies.z4.web.core.windows.net
How Do Electrons Flow In A Voltaic Cell Electrodes In Voltaic Cell the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Electrodes In Voltaic Cell.
From www.sliderbase.com
Electrochemical Terminology Electrodes In Voltaic Cell voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. The structure of a voltaic cell. There are two electrodes in a voltaic cell, one in. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. This means that the oxidation. Electrodes In Voltaic Cell.
From cider.uoregon.edu
Electrochemcial Cell Demonstration Voltaic Cell Zinc/Copper CIDER Electrodes In Voltaic Cell the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. in a. Electrodes In Voltaic Cell.
From circuitlibrarylinty.z13.web.core.windows.net
Voltaic Cell Diagram Electrodes In Voltaic Cell The structure of a voltaic cell. an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. There are two electrodes in a voltaic cell, one in. in a galvanic (voltaic) cell, the energy from a spontaneous. Electrodes In Voltaic Cell.
From stock.adobe.com
Vector scientific illustration of the electrolysis processes. Set of Electrodes In Voltaic Cell The structure of a voltaic cell. There are two electrodes in a voltaic cell, one in. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. an electrochemical cell in. Electrodes In Voltaic Cell.
From userlistyaadhibiting.z13.web.core.windows.net
Simple Cell Diagram In Electricity Electrodes In Voltaic Cell voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. There are two electrodes in a voltaic cell, one in. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an. Electrodes In Voltaic Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281515 Electrodes In Voltaic Cell voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. There are two electrodes in a voltaic cell, one in. in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. This means that the oxidation will. the galvanic cell, or called voltaic cell, is an electrochemical. Electrodes In Voltaic Cell.
From byjus.com
Explain the construction and working of a voltaic cell. Electrodes In Voltaic Cell the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. This means that the oxidation will. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas. Electrodes In Voltaic Cell.
From labsuppliesusa.com
Voltaic Cell Supplied With 2 Electrodes KLM Bio Scientific Electrodes In Voltaic Cell an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. The structure of a voltaic cell. the galvanic. Electrodes In Voltaic Cell.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical Electrodes In Voltaic Cell in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is. There are two electrodes in a voltaic cell, one in. The structure of a voltaic cell. a voltaic cell is an electrochemical. Electrodes In Voltaic Cell.
From overallscience.com
Voltaic Cell, Its Construction and Defects Overall Science Electrodes In Voltaic Cell a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is. The structure of a voltaic. Electrodes In Voltaic Cell.
From slideplayer.com
Voltaic Cells Aim To identify the components and explain the functions Electrodes In Voltaic Cell There are two electrodes in a voltaic cell, one in. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is. voltaic cells, also known as galvanic cells, are fundamental. Electrodes In Voltaic Cell.
From theedge.com.hk
Chemistry How To Electrochemical Cell The Edge Electrodes In Voltaic Cell the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy. Electrodes In Voltaic Cell.
From byjus.com
The electrolyte of simple voltaic cell is Electrodes In Voltaic Cell The structure of a voltaic cell. There are two electrodes in a voltaic cell, one in. an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. voltaic cells, also known as galvanic cells, are fundamental devices. Electrodes In Voltaic Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrodes In Voltaic Cell There are two electrodes in a voltaic cell, one in. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. The structure of a voltaic cell. in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. This means that the oxidation will. an electrochemical cell in. Electrodes In Voltaic Cell.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Electrodes In Voltaic Cell voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. The structure of a voltaic cell. an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell.. Electrodes In Voltaic Cell.
From www.sciencephoto.com
Voltaic Cell and Electrodes Stock Image C027/7935 Science Photo Electrodes In Voltaic Cell in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. There are two electrodes in a voltaic cell, one in. a voltaic cell is an electrochemical. Electrodes In Voltaic Cell.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Electrodes In Voltaic Cell The structure of a voltaic cell. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell.. Electrodes In Voltaic Cell.
From chem.libretexts.org
5.4 Day 39 Voltaic Cells, HalfCell Potentials Chemistry LibreTexts Electrodes In Voltaic Cell voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is. This means that the oxidation will. an electrochemical cell in which the chemistry is spontaneous is called a voltaic. Electrodes In Voltaic Cell.
From www.slideserve.com
PPT Chemistry 30 Chapter 14 PowerPoint Presentation, free download Electrodes In Voltaic Cell This means that the oxidation will. voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic. Electrodes In Voltaic Cell.
From usermanualtractors.z1.web.core.windows.net
Cathode In Electrochemical Cell Electrodes In Voltaic Cell a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. The structure of a voltaic cell. in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. There are two. Electrodes In Voltaic Cell.
From brainly.com
Label each half cell in this diagram of a voltaic cell to identify Electrodes In Voltaic Cell the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. The structure of a voltaic cell. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell.. Electrodes In Voltaic Cell.
From chem.libretexts.org
Voltaic Cells Chemistry LibreTexts Electrodes In Voltaic Cell in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. There are two electrodes in a voltaic cell, one in. an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical. Electrodes In Voltaic Cell.
From www.youtube.com
9.4.1 Explain how a redox reaction is used to produce electricity in a Electrodes In Voltaic Cell an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. This means that the oxidation will. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from.. Electrodes In Voltaic Cell.
From cider.uoregon.edu
Voltaic Galvanic Cell (Electrochemical Cell) Simulation AACT CIDER Electrodes In Voltaic Cell There are two electrodes in a voltaic cell, one in. voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy. Electrodes In Voltaic Cell.
From slideplayer.com
Voltaic Cells Aim To identify the components and explain the functions Electrodes In Voltaic Cell in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. The structure of a voltaic cell. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts. Electrodes In Voltaic Cell.
From www.slideserve.com
PPT Electrochemical Cells (voltaic cells) PowerPoint Presentation Electrodes In Voltaic Cell the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. There are two electrodes in a voltaic cell, one in. in a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity,. Electrodes In Voltaic Cell.
From www.youtube.com
Ch. 203 Inert Electrodes/Voltaic Cells YouTube Electrodes In Voltaic Cell voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. in a. Electrodes In Voltaic Cell.
From www.slideserve.com
PPT Voltaic Cells PowerPoint Presentation, free download ID3061019 Electrodes In Voltaic Cell in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. The structure of a voltaic cell. This means that the oxidation will. There are two electrodes in a voltaic cell, one in. in. Electrodes In Voltaic Cell.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Electrodes In Voltaic Cell voltaic cells, also known as galvanic cells, are fundamental devices in electrochemistry that convert chemical energy into. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. There are two electrodes in a voltaic cell, one in. This means that the oxidation will. a voltaic cell is. Electrodes In Voltaic Cell.
From www.echemi.com
Where do negative ions migrate from the salt bridge to which electrode Electrodes In Voltaic Cell an electrochemical cell in which the chemistry is spontaneous is called a voltaic cell. There are two electrodes in a voltaic cell, one in. in a voltaic cell, the oxidation and reduction of metals occurs at the electrodes. The structure of a voltaic cell. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction. Electrodes In Voltaic Cell.
From www.numerade.com
SOLVED Label each half cell in this diagram of a voltaic cell to Electrodes In Voltaic Cell the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. This means that the oxidation will. a voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. There are two electrodes in a voltaic cell, one in. in a voltaic cell,. Electrodes In Voltaic Cell.