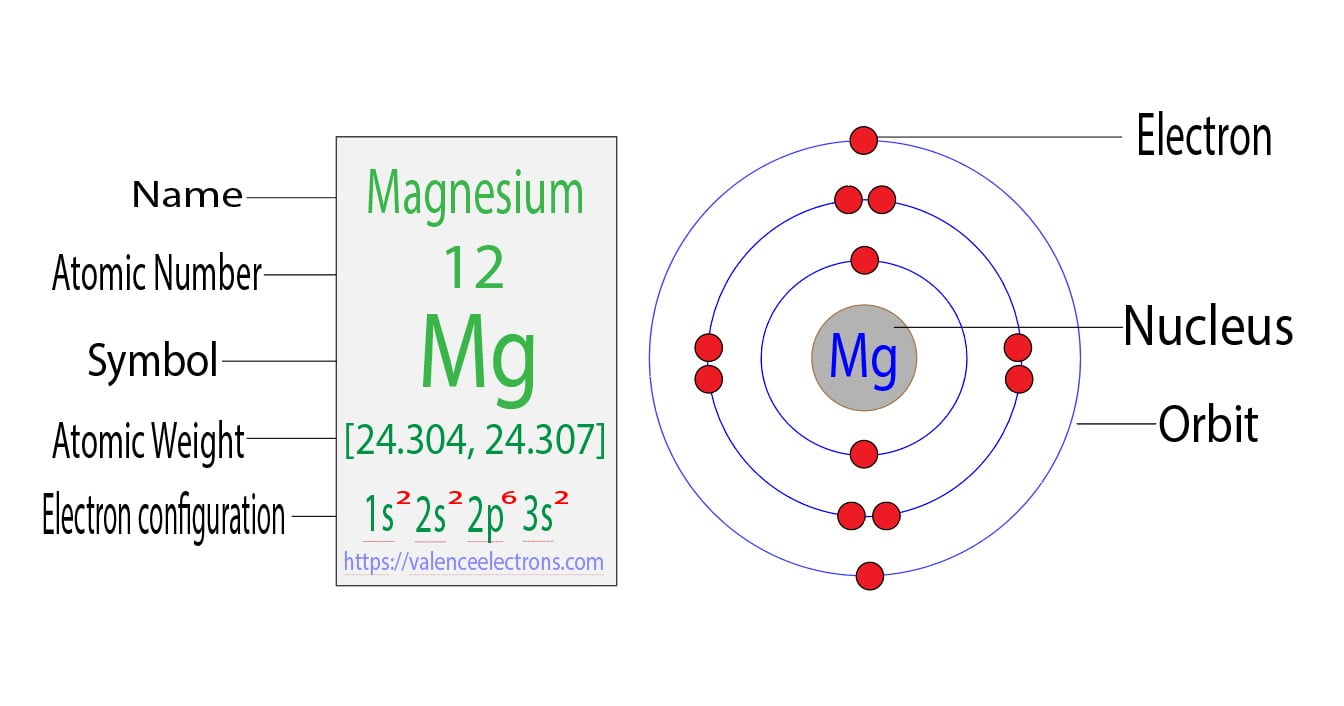
Magnesium Lose Ion . A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions (cations). for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: In this ocr gcse chemistry study guide, we'll go through. metal atoms lose electrons from their outer shell when they form ions: metals form positive ions (cations). metals form positive ions (cations). The ions are positive, because they have more. A magnesium atom must lose two electrons to have the same number electrons as an. 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is. A magnesium atom must lose two electrons to have the same number electrons as an.
from valenceelectrons.com
metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. A magnesium atom must lose two electrons to have the same number electrons as an. In this ocr gcse chemistry study guide, we'll go through. A magnesium atom must lose two electrons to have the same number electrons as an. 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is. metals form positive ions (cations). for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: The ions are positive, because they have more. metals form positive ions (cations).
Magnesium Electron Configuration Aufbau & Bohr Model
Magnesium Lose Ion metal atoms lose electrons from their outer shell when they form ions: metal atoms lose electrons from their outer shell when they form ions: The ions are positive, because they have more. A magnesium atom must lose two electrons to have the same number electrons as an. In this ocr gcse chemistry study guide, we'll go through. 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is. metals form positive ions (cations). for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: A magnesium atom must lose two electrons to have the same number electrons as an. A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions (cations). metals form positive ions (cations).
From www.youtube.com
How to Find the Ionic Charge for Magnesium (Mg) YouTube Magnesium Lose Ion metals form positive ions (cations). metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. A magnesium atom must lose two electrons to have the same number electrons as an. for the ionic compound between mg 2 + ions and cl − ions, we now consider the. Magnesium Lose Ion.
From chemistry291.blogspot.com
Oxidation of Magnesium (Mg) What Is the Oxidation State of Magnesium? Magnesium Lose Ion 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is. metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. A magnesium atom must lose two electrons to have the same number electrons. Magnesium Lose Ion.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Magnesium Lose Ion In this ocr gcse chemistry study guide, we'll go through. The ions are positive, because they have more. A magnesium atom must lose two electrons to have the same number electrons as an. A magnesium atom must lose two electrons to have the same number electrons as an. 93 rows — — when atoms gain electron/s, the negatively charged. Magnesium Lose Ion.
From www.difference.wiki
Magnesium Atom vs. Magnesium Ion What’s the Difference? Magnesium Lose Ion metals form positive ions (cations). metal atoms lose electrons from their outer shell when they form ions: A magnesium atom must lose two electrons to have the same number electrons as an. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: . Magnesium Lose Ion.
From www.researchgate.net
Schematic illustrations of the behavior of the magnesium ions at the Magnesium Lose Ion The ions are positive, because they have more. A magnesium atom must lose two electrons to have the same number electrons as an. 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is. metals form positive ions (cations). In this ocr gcse chemistry. Magnesium Lose Ion.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Lose Ion The ions are positive, because they have more. metals form positive ions (cations). metal atoms lose electrons from their outer shell when they form ions: metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. A magnesium atom must lose two electrons to have the same number. Magnesium Lose Ion.
From www.flexiprep.com
NCERT Class 9 Science Solutions Chapter 3 Atoms and Molecules Part 9 Magnesium Lose Ion A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions (cations). metal atoms lose electrons from their outer shell when they form ions: A magnesium atom must lose two electrons to have the same number electrons as an. 93 rows — — when atoms gain electron/s, the negatively. Magnesium Lose Ion.
From brainly.in
EXPLAIN WHY MAGNESIUM FORMS MG ION Brainly.in Magnesium Lose Ion metals form positive ions (cations). In this ocr gcse chemistry study guide, we'll go through. The ions are positive, because they have more. A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions (cations). metals form positive ions (cations). A magnesium atom must lose two electrons to have. Magnesium Lose Ion.
From www.researchgate.net
Amounts of magnesium ions released from the magnesium ion modified Magnesium Lose Ion metal atoms lose electrons from their outer shell when they form ions: metals form positive ions (cations). In this ocr gcse chemistry study guide, we'll go through. A magnesium atom must lose two electrons to have the same number electrons as an. The ions are positive, because they have more. metals form positive ions (cations). for. Magnesium Lose Ion.
From mungfali.com
Magnesium Orbital Diagram Magnesium Lose Ion A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. A magnesium atom must lose two electrons to have the same number electrons as an. for the ionic compound between mg 2 +. Magnesium Lose Ion.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Lose Ion metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. The ions are positive, because they have more. metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. for the ionic compound between mg 2 + ions. Magnesium Lose Ion.
From www.youtube.com
Magnesium loses electrons successively to form Mg+, Mg2+ and Mg3+ ions Magnesium Lose Ion The ions are positive, because they have more. metal atoms lose electrons from their outer shell when they form ions: for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: metals form positive ions (cations). metals form positive ions (cations). In this. Magnesium Lose Ion.
From www.nagwa.com
Vidéo question Écrire la formule chimique de l’ion magnésium Nagwa Magnesium Lose Ion A magnesium atom must lose two electrons to have the same number electrons as an. A magnesium atom must lose two electrons to have the same number electrons as an. The ions are positive, because they have more. metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. . Magnesium Lose Ion.
From byjus.com
By the transfer of electrons, illustrate the formation of bond in Magnesium Lose Ion A magnesium atom must lose two electrons to have the same number electrons as an. A magnesium atom must lose two electrons to have the same number electrons as an. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: metals form positive ions. Magnesium Lose Ion.
From valenceelectrons.com
Magnesium Electron Configuration Aufbau & Bohr Model Magnesium Lose Ion metal atoms lose electrons from their outer shell when they form ions: metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is.. Magnesium Lose Ion.
From periodictable.me
Magnesium Valence Electron Magnesium Valency (Mg) with Dot Diagram Magnesium Lose Ion for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions (cations). The ions are positive, because they have more. 93 rows — — when. Magnesium Lose Ion.
From www.tuttee.co
CHEM Ionic Bonding chemistry ionic bond ionic compounds ions Magnesium Lose Ion for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: metal atoms lose electrons from their outer shell when they form ions: In this ocr gcse chemistry study guide, we'll go through. metals form positive ions (cations). A magnesium atom must lose two. Magnesium Lose Ion.
From circuitlibsmoring.z21.web.core.windows.net
Magnesium Ion Atom Diagram Magnesium Lose Ion A magnesium atom must lose two electrons to have the same number electrons as an. A magnesium atom must lose two electrons to have the same number electrons as an. metal atoms lose electrons from their outer shell when they form ions: for the ionic compound between mg 2 + ions and cl − ions, we now consider. Magnesium Lose Ion.
From manualfixageundeployed.z13.web.core.windows.net
Magnesium Atomic Structure Diagram Magnesium Lose Ion A magnesium atom must lose two electrons to have the same number electrons as an. 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is. A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive. Magnesium Lose Ion.
From mungfali.com
Magnesium Orbital Diagram Magnesium Lose Ion metals form positive ions (cations). The ions are positive, because they have more. metals form positive ions (cations). metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact. Magnesium Lose Ion.
From www.showme.com
Magnesium + Oxygen Ionic Bonding Science, Chemistry ShowMe Magnesium Lose Ion A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions (cations). metal atoms lose electrons from their outer shell when they form ions: A magnesium atom must lose two electrons to have the same number electrons as an. In this ocr gcse chemistry study guide, we'll go through. . Magnesium Lose Ion.
From study.com
How many electrons does magnesium lose and nitrogen need to form Mg3N2 Magnesium Lose Ion 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is. metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. for the ionic compound between mg 2 + ions and cl −. Magnesium Lose Ion.
From de.dreamstime.com
Magnesiumatom Auf Einem Weißen Hintergrund Stock Abbildung Magnesium Lose Ion The ions are positive, because they have more. 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is. metals form positive ions (cations). metal atoms lose electrons from their outer shell when they form ions: A magnesium atom must lose two electrons. Magnesium Lose Ion.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Lose Ion A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions (cations). 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is. In this ocr gcse chemistry study guide, we'll go through. metal atoms. Magnesium Lose Ion.
From la.wikipedia.org
FasciculusElectron shell 012 magnesium.png Vicipaedia Magnesium Lose Ion for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: The ions are positive, because they have more. metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. metal atoms lose electrons from. Magnesium Lose Ion.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Lose Ion A magnesium atom must lose two electrons to have the same number electrons as an. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: The ions are positive, because they have more. 93 rows — — when atoms gain electron/s, the negatively charged. Magnesium Lose Ion.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Lose Ion metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. for the ionic compound between mg 2 + ions and cl − ions, we now consider the. Magnesium Lose Ion.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.6 4 Ionic Bonds Dot Magnesium Lose Ion metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions (cations). 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is. The ions are positive, because they have. Magnesium Lose Ion.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Lose Ion In this ocr gcse chemistry study guide, we'll go through. A magnesium atom must lose two electrons to have the same number electrons as an. metal atoms lose electrons from their outer shell when they form ions: metals form positive ions (cations). The ions are positive, because they have more. metals form positive ions (cations). metals. Magnesium Lose Ion.
From socialyme.com
Comment Choisir Son Magnésium ? Tout ce qu'il faut savoir sur le Mg Magnesium Lose Ion metals form positive ions (cations). metals form positive ions (cations). metals form positive ions (cations). for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: A magnesium atom must lose two electrons to have the same number electrons as an. The ions. Magnesium Lose Ion.
From www.toppr.com
When magnesium makes an ionic bond with oxygen it loses two electrons Magnesium Lose Ion for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: A magnesium atom must lose two electrons to have the same number electrons as an. A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions. Magnesium Lose Ion.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.40 Draw DotandCross Diagrams to Show the Magnesium Lose Ion metal atoms lose electrons from their outer shell when they form ions: for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have different magnitudes: metals form positive ions (cations). metals form positive ions (cations). metals form positive ions (cations). A magnesium atom must. Magnesium Lose Ion.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Magnesium Lose Ion metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions (cations). 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is. metal atoms lose electrons from their. Magnesium Lose Ion.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Lose Ion metals form positive ions (cations). In this ocr gcse chemistry study guide, we'll go through. metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an. for the ionic compound between mg 2 + ions and cl − ions, we now consider the fact that the charges have. Magnesium Lose Ion.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 Magnesium Lose Ion A magnesium atom must lose two electrons to have the same number electrons as an. metals form positive ions (cations). metals form positive ions (cations). The ions are positive, because they have more. 93 rows — — when atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion. Magnesium Lose Ion.