Tin(Iv) Hydrogen Sulfide . Note, hydrogen can not lose its only. Finally, the prospects and opportunities in. Tin (iv) sulfide is a compound with the formula sn s 2. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. This represents the formula snf 2, which is more properly named tin(ii) fluoride. With only a few exceptions, these metals are usually transition metals or actinides. This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java. The other fluoride of tin is snf 4, which was previously called stannic. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation.
from www.chegg.com
The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. Tin (iv) sulfide is a compound with the formula sn s 2. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java. Note, hydrogen can not lose its only. The other fluoride of tin is snf 4, which was previously called stannic. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. This represents the formula snf 2, which is more properly named tin(ii) fluoride. With only a few exceptions, these metals are usually transition metals or actinides. Finally, the prospects and opportunities in. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications.
Solved An unknown compound contains only C,H, and O.
Tin(Iv) Hydrogen Sulfide The other fluoride of tin is snf 4, which was previously called stannic. Finally, the prospects and opportunities in. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. With only a few exceptions, these metals are usually transition metals or actinides. The other fluoride of tin is snf 4, which was previously called stannic. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. Tin (iv) sulfide is a compound with the formula sn s 2. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Note, hydrogen can not lose its only.
From slideplayer.com
What’s in a name?. ppt download Tin(Iv) Hydrogen Sulfide The other fluoride of tin is snf 4, which was previously called stannic. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. With only a few exceptions, these metals are usually transition metals or actinides.. Tin(Iv) Hydrogen Sulfide.
From www.ossila.com
SnS2, Tin(IV) Sulfide Powder & Crystal CAS Number 1315011 Ossila Tin(Iv) Hydrogen Sulfide Tin (iv) sulfide is a compound with the formula sn s 2. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. Note, hydrogen can not lose its only. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. Finally, the prospects and opportunities in. This. Tin(Iv) Hydrogen Sulfide.
From vdocuments.mx
Selective detection of hydrogen sulfide using copper oxidedoped tin Tin(Iv) Hydrogen Sulfide Note, hydrogen can not lose its only. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. With only a few exceptions, these metals are usually transition metals or actinides. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be. Tin(Iv) Hydrogen Sulfide.
From www.youtube.com
How to Write the Formula for Tin (IV) oxide YouTube Tin(Iv) Hydrogen Sulfide With only a few exceptions, these metals are usually transition metals or actinides. Note, hydrogen can not lose its only. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. Finally, the prospects and opportunities in. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be. Tin(Iv) Hydrogen Sulfide.
From www.t3db.ca
T3DB Tin(IV) sulfide Tin(Iv) Hydrogen Sulfide The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. Tin (iv) sulfide is a compound with the formula sn s 2. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. The review includes the properties and synthesis of tin sulfides and also. Tin(Iv) Hydrogen Sulfide.
From www.slideserve.com
PPT Calcium Sulfide PowerPoint Presentation, free download ID3730769 Tin(Iv) Hydrogen Sulfide The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. The other fluoride of tin is snf 4, which was previously called stannic. Note, hydrogen can not lose its only. Tin (iv) sulfide is a compound with the formula sn s 2. If it is an acid, we base it's name on the ionic compound it. Tin(Iv) Hydrogen Sulfide.
From eureka.patsnap.com
Tindoped (IV) sulfide lanthanum pigment and preparation method thereof Tin(Iv) Hydrogen Sulfide The other fluoride of tin is snf 4, which was previously called stannic. Tin (iv) sulfide is a compound with the formula sn s 2. Finally, the prospects and opportunities in. Note, hydrogen can not lose its only. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. With only a few exceptions, these metals are. Tin(Iv) Hydrogen Sulfide.
From butchixanh.edu.vn
Top 7+ tin iv sulfide formula Latest Bút Chì Xanh Tin(Iv) Hydrogen Sulfide The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java. The other fluoride of tin is snf 4, which was previously called stannic. This represents. Tin(Iv) Hydrogen Sulfide.
From www.nanochemazone.com
Tin(IV) Sulfide Powder Low Price 1 highly pure Nanochemazone Tin(Iv) Hydrogen Sulfide This represents the formula snf 2, which is more properly named tin(ii) fluoride. Tin (iv) sulfide is a compound with the formula sn s 2. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. With only a few exceptions, these metals are usually transition metals or actinides.. Tin(Iv) Hydrogen Sulfide.
From www.semanticscholar.org
Figure 1 from A critical role of hydrogen sulfide evolution during Tin(Iv) Hydrogen Sulfide With only a few exceptions, these metals are usually transition metals or actinides. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java.. Tin(Iv) Hydrogen Sulfide.
From www.mdpi.com
Molecules Free FullText Hydrogen Sulfide Biology and Its Role in Tin(Iv) Hydrogen Sulfide The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. Note, hydrogen can not lose its only. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Finally, the prospects and opportunities in.. Tin(Iv) Hydrogen Sulfide.
From www.chegg.com
Solved An unknown compound contains only C,H, and O. Tin(Iv) Hydrogen Sulfide If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. With only a few exceptions, these metals are usually transition metals or actinides. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. This structure is also available as a 2d mol file or. Tin(Iv) Hydrogen Sulfide.
From www2.mdpi.com
Applied Sciences Free FullText A Review of the Synthesis Tin(Iv) Hydrogen Sulfide Finally, the prospects and opportunities in. Note, hydrogen can not lose its only. With only a few exceptions, these metals are usually transition metals or actinides. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be. Tin(Iv) Hydrogen Sulfide.
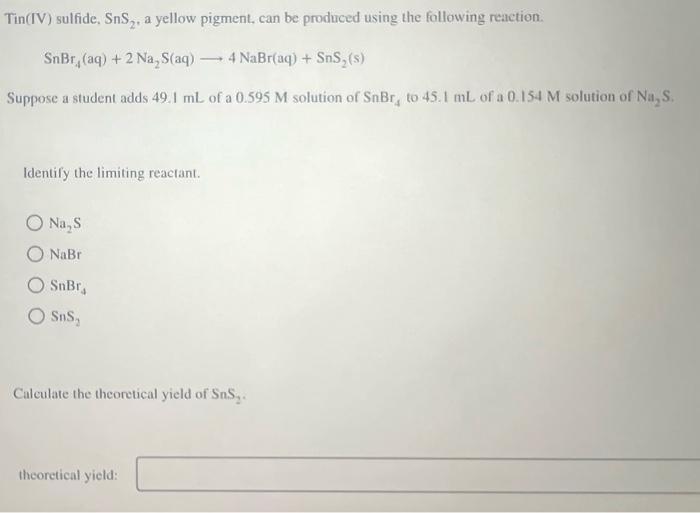
From www.chegg.com
Solved Tin(IV) sulfide, SnS2, a yellow pigment, can Tin(Iv) Hydrogen Sulfide Finally, the prospects and opportunities in. This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. The other fluoride. Tin(Iv) Hydrogen Sulfide.
From www.youtube.com
How to write chemical formula of tin (lV) Sulfideformula of stannic Tin(Iv) Hydrogen Sulfide The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Note, hydrogen can not lose its only. The other fluoride of. Tin(Iv) Hydrogen Sulfide.
From encyclopedia.pub
The Role of Hydrogen Sulfide in Macrophage Biology Encyclopedia MDPI Tin(Iv) Hydrogen Sulfide Finally, the prospects and opportunities in. Note, hydrogen can not lose its only. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. This represents the formula snf 2, which is more properly named tin(ii) fluoride. With only a few exceptions, these metals are usually transition metals or actinides. This. Tin(Iv) Hydrogen Sulfide.
From studylib.net
SDS for Tin (IV) Sulfide Tin(Iv) Hydrogen Sulfide With only a few exceptions, these metals are usually transition metals or actinides. Tin (iv) sulfide is a compound with the formula sn s 2. This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. This represents the formula snf 2, which is more properly named tin(ii) fluoride. The compound crystallizes in the. Tin(Iv) Hydrogen Sulfide.
From www.dreamstime.com
SnS2 Tin (IV) Sulfide stock image. Image of flask 296779805 Tin(Iv) Hydrogen Sulfide This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. Finally, the prospects and opportunities in. Note, hydrogen can not lose its only. Tin (iv) sulfide is a compound with the formula sn s 2. This represents the formula snf 2, which is more properly named tin(ii) fluoride. This structure is also available. Tin(Iv) Hydrogen Sulfide.
From www.youtube.com
How to Write the Formula for Tin (IV) sulfide YouTube Tin(Iv) Hydrogen Sulfide This represents the formula snf 2, which is more properly named tin(ii) fluoride. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java. This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. Tin (iv) sulfide is a. Tin(Iv) Hydrogen Sulfide.
From journals.iucr.org
(IUCr) Redetermination of the crystal structure of diμ2hydroxidobis Tin(Iv) Hydrogen Sulfide The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. The other fluoride of tin is snf 4, which was previously called stannic. This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. If it is an acid, we base it's name on the ionic compound it would form. Tin(Iv) Hydrogen Sulfide.
From heegermaterials.com
Tin (IV) Sulfide SnS Heeger Materials Tin(Iv) Hydrogen Sulfide With only a few exceptions, these metals are usually transition metals or actinides. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. Note, hydrogen can not lose its only. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. The other fluoride of. Tin(Iv) Hydrogen Sulfide.
From zegmetal.com
Tin Disulfide SnS2 Tin IV Sulfide Powder CAS 1315101 Semiconductor Tin(Iv) Hydrogen Sulfide This represents the formula snf 2, which is more properly named tin(ii) fluoride. If it is an acid, we base it's name on the ionic compound it would form if hydrogen could be a cation. Note, hydrogen can not lose its only. Finally, the prospects and opportunities in. This represents the formula snf 2, which is also named tin(ii) fluoride. Tin(Iv) Hydrogen Sulfide.
From www.numerade.com
SOLVED For the equation SnO2 + 2H2 Sn + 2H2O, tin (IV) oxide reacts Tin(Iv) Hydrogen Sulfide The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. Note, hydrogen can not lose its only. This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be. Tin(Iv) Hydrogen Sulfide.
From www.anyrgb.com
Polysulfide, ferrocene, tis, h 5, c 5, hydrogen Sulfide, sulfide Tin(Iv) Hydrogen Sulfide Note, hydrogen can not lose its only. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d. Tin(Iv) Hydrogen Sulfide.
From supply.coreandmain.com
Hach Hydrogen Sulfide Test Vial, 2532800 Tin(Iv) Hydrogen Sulfide The other fluoride of tin is snf 4, which was previously called stannic. Tin (iv) sulfide is a compound with the formula sn s 2. This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Note, hydrogen can not lose. Tin(Iv) Hydrogen Sulfide.
From aulickchemical.com
Understanding Hydrogen Sulfide (H2S) Aulick Chemical Solutions Tin(Iv) Hydrogen Sulfide This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. Tin (iv) sulfide is a compound with the formula sn s 2. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. Finally, the prospects and opportunities in. This structure is also available. Tin(Iv) Hydrogen Sulfide.
From www.tandfonline.com
Full article Influence of Dilution Upon the UltravioletVisible Peak Tin(Iv) Hydrogen Sulfide This represents the formula snf 2, which is more properly named tin(ii) fluoride. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. With only a few exceptions, these metals are usually transition metals or actinides. Note, hydrogen can not lose its only. The review includes the properties and synthesis of tin sulfides and also delivers. Tin(Iv) Hydrogen Sulfide.
From www.researchgate.net
Scheme 2. Oxidations of 2[OTf] leading to tin(IV) salts 3[OTf] and 4 Tin(Iv) Hydrogen Sulfide The other fluoride of tin is snf 4, which was previously called stannic. This represents the formula snf 2, which is more properly named tin(ii) fluoride. With only a few exceptions, these metals are usually transition metals or actinides. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. Finally, the prospects and opportunities in. If. Tin(Iv) Hydrogen Sulfide.
From heegermaterials.com
Tin(IV) Sulfide (SnS2) Evaporation Material Heeger Materials Tin(Iv) Hydrogen Sulfide The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. Tin (iv) sulfide is a compound with the formula sn s 2. This represents the formula snf 2, which is more properly named tin(ii) fluoride. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. Finally,. Tin(Iv) Hydrogen Sulfide.
From www.mdpi.com
Molecules Free FullText Tin(II) and Tin(IV) Complexes Tin(Iv) Hydrogen Sulfide This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java. If it is an acid, we base it's name on the ionic compound it would form if hydrogen. Tin(Iv) Hydrogen Sulfide.
From www.researchgate.net
Schematic representation of tin sulfide polymorph (πSnS) nanoparticles Tin(Iv) Hydrogen Sulfide This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. This represents the formula snf 2, which is more properly named tin(ii) fluoride. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. With only a few exceptions, these metals are usually transition metals or actinides. Note, hydrogen can. Tin(Iv) Hydrogen Sulfide.
From labchem-wako.fujifilm.com
1315011・硫化すず(IV)・Tin(IV) Sulfide・20021121【詳細情報】|【合成・材料】|試薬富士フイルム和光純薬 Tin(Iv) Hydrogen Sulfide The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. Finally, the prospects and opportunities in. The other fluoride of tin is snf 4, which was previously called stannic. This represents the formula snf 2, which is also named tin(ii) fluoride following the more current convention. Tin (iv) sulfide is. Tin(Iv) Hydrogen Sulfide.
From www.attelements.com
Tin(IV) Sulfide (SnS2) Evaporation Material Exporter China Tin(Iv) Hydrogen Sulfide Tin (iv) sulfide is a compound with the formula sn s 2. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. Finally, the prospects and opportunities in. This represents the formula snf 2, which is more properly named tin(ii) fluoride. Note, hydrogen can not lose its only. The compound. Tin(Iv) Hydrogen Sulfide.
From www.researchgate.net
(PDF) Chemical vapor deposition of tin sulfide from Tin(Iv) Hydrogen Sulfide The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java. With only a few exceptions, these metals are usually transition metals or actinides. The other. Tin(Iv) Hydrogen Sulfide.
From www.alamy.com
Hydrogen sulfide, ballandstick model, molecular and chemical formula Tin(Iv) Hydrogen Sulfide Finally, the prospects and opportunities in. The compound crystallizes in the cadmium iodide motif, with the sn (iv) situated in. The review includes the properties and synthesis of tin sulfides and also delivers exciting progress in various fields of applications. Note, hydrogen can not lose its only. Tin (iv) sulfide is a compound with the formula sn s 2. With. Tin(Iv) Hydrogen Sulfide.