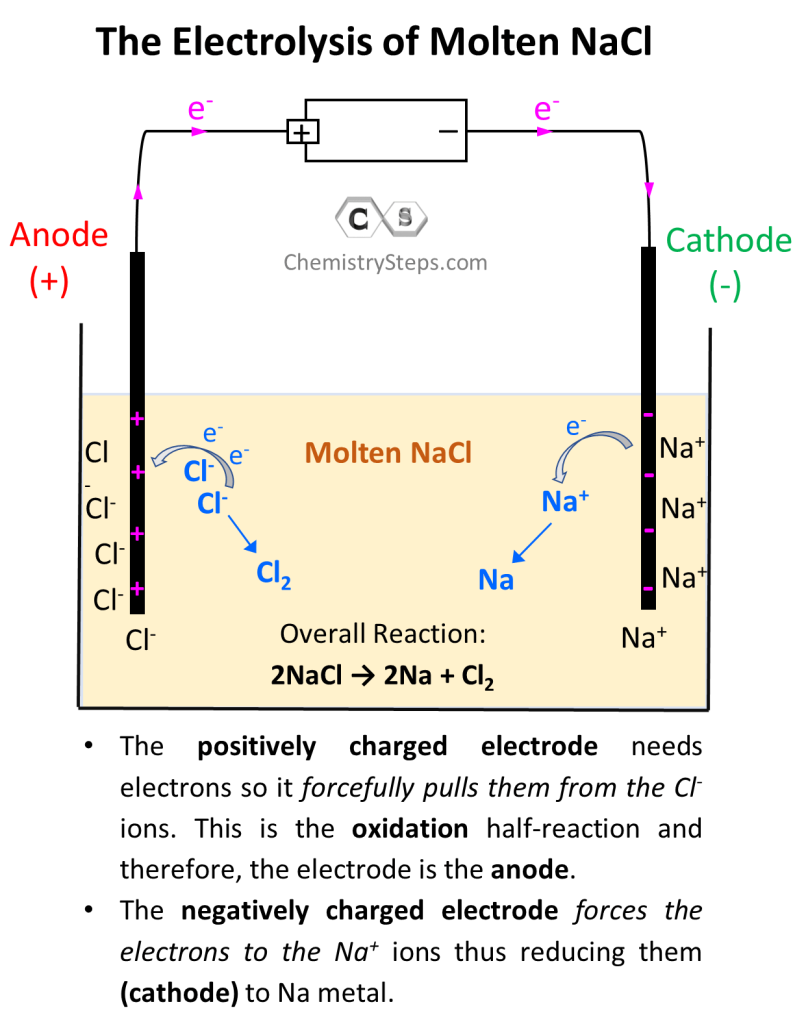
Electrolysis Of Table Salt Element . There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. Electrolysis of molten sodium chloride. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Salts are ionic compounds which can be electrolysed when they are in the molten state. The products of electrolysis can be predicted for a given. Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. Gases may be formed at the electrodes. Electrolysis of aqueous salts •during electrolysis of the aqueous salt, e.g. Cacl 2, hydrogen gas is produced at the cathode, not the metallic.
from general.chemistrysteps.com
Electrolysis of aqueous salts •during electrolysis of the aqueous salt, e.g. The products of electrolysis can be predicted for a given. Salts are ionic compounds which can be electrolysed when they are in the molten state. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Electrolysis involves using electricity to break down electrolytes to form elements. There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. Gases may be formed at the electrodes. Cacl 2, hydrogen gas is produced at the cathode, not the metallic. Electrolysis of molten sodium chloride.
Electrolysis Chemistry Steps
Electrolysis Of Table Salt Element Electrolysis of aqueous salts •during electrolysis of the aqueous salt, e.g. Gases may be formed at the electrodes. Electrolysis of aqueous salts •during electrolysis of the aqueous salt, e.g. Salts are ionic compounds which can be electrolysed when they are in the molten state. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Cacl 2, hydrogen gas is produced at the cathode, not the metallic. The products of electrolysis can be predicted for a given. Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis of molten sodium chloride. There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts.
From www.researchgate.net
Schematic illustration of molten salt electrolysis using an inert anode... Download Scientific Electrolysis Of Table Salt Element Cacl 2, hydrogen gas is produced at the cathode, not the metallic. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. There are two important points to make about these two. Electrolysis Of Table Salt Element.
From mungfali.com
Sodium Chloride Electrolysis Equation Electrolysis Of Table Salt Element Salts are ionic compounds which can be electrolysed when they are in the molten state. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Cacl 2, hydrogen gas is produced at the cathode, not the metallic. Electrolysis of molten sodium chloride. Molten (liquid) sodium chloride can be electrolyzed. Electrolysis Of Table Salt Element.
From studymind.co.uk
Electrolysis of Aqueous Solutions (GCSE Chemistry) Study Mind Electrolysis Of Table Salt Element Gases may be formed at the electrodes. Salts are ionic compounds which can be electrolysed when they are in the molten state. The products of electrolysis can be predicted for a given. There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. Electrolysis involves using electricity to break down. Electrolysis Of Table Salt Element.
From www.slideserve.com
PPT Electrolysis of Salt Water PowerPoint Presentation, free download ID508875 Electrolysis Of Table Salt Element In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. The products of electrolysis can be predicted for a given. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. Electrolysis involves using electricity to. Electrolysis Of Table Salt Element.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Electrolysis Of Table Salt Element Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. The products of electrolysis can be predicted for a given. Electrolysis involves using electricity to. Electrolysis Of Table Salt Element.
From uen.pressbooks.pub
Electrolysis Introductory Chemistry Electrolysis Of Table Salt Element Electrolysis of molten sodium chloride. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Electrolysis involves using electricity to break down electrolytes to form elements. Cacl 2, hydrogen gas is produced at the cathode, not the metallic. The products of electrolysis can be predicted for a given. Salts. Electrolysis Of Table Salt Element.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Electrolysis Of Table Salt Element Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. Salts are ionic compounds which can be electrolysed when they are in the molten state. There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general.. Electrolysis Of Table Salt Element.
From www.slideserve.com
PPT Electrolysis of salt 3 PowerPoint Presentation, free download ID4639459 Electrolysis Of Table Salt Element Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. Electrolysis of molten sodium chloride. Electrolysis of aqueous salts •during electrolysis of the aqueous salt,. Electrolysis Of Table Salt Element.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Electrolysis Of Table Salt Element There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. Electrolysis involves using electricity to break down electrolytes to form elements. Salts are ionic compounds. Electrolysis Of Table Salt Element.
From www.pinterest.co.uk
Understanding purpose of salt bridge Electrochemistry, Chemistry education, Science chemistry Electrolysis Of Table Salt Element Gases may be formed at the electrodes. The products of electrolysis can be predicted for a given. Electrolysis of molten sodium chloride. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. In this explainer, we will learn how to predict the products of electrolysis of. Electrolysis Of Table Salt Element.
From www.youtube.com
Electrolysis of Sodium Chloride Electrochemistry YouTube Electrolysis Of Table Salt Element Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. Gases may be formed at the electrodes. Electrolysis of aqueous salts •during electrolysis of the aqueous salt, e.g. Electrolysis of molten sodium chloride. In this explainer, we will learn how to predict the products of electrolysis. Electrolysis Of Table Salt Element.
From chemicalengineeringworld.com
Electrolysis Overview Chemical Engineering World Electrolysis Of Table Salt Element In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine,. Electrolysis Of Table Salt Element.
From study.com
Electrolysis of Aqueous Solutions Lesson Electrolysis Of Table Salt Element The products of electrolysis can be predicted for a given. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. Salts are ionic compounds which can be electrolysed when they are in the molten state. Gases may be formed at the electrodes. Electrolysis of aqueous salts. Electrolysis Of Table Salt Element.
From www.slideserve.com
PPT Electrolysis of salt 3 PowerPoint Presentation, free download ID5480714 Electrolysis Of Table Salt Element Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Gases may be formed at the electrodes. Cacl 2, hydrogen gas is produced at the cathode, not the metallic. Salts are ionic compounds which can. Electrolysis Of Table Salt Element.
From www.studocu.com
Chap 19 pt7 Electrolysis intro and Molten salts Chapter 19 Electrochemistry Electrolysis Electrolysis Of Table Salt Element Salts are ionic compounds which can be electrolysed when they are in the molten state. Cacl 2, hydrogen gas is produced at the cathode, not the metallic. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Electrolysis of molten sodium chloride. Electrolysis involves using electricity to break down. Electrolysis Of Table Salt Element.
From www.coursehero.com
[Solved] . Electrolysis of molten salt Exercises Draw the electrolytic... Course Hero Electrolysis Of Table Salt Element In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Cacl 2, hydrogen gas is produced at the cathode, not the metallic. There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. Electrolysis of an aqueous solution of. Electrolysis Of Table Salt Element.
From gbu-presnenskij.ru
Faraday's Laws Of Electrolysis Equation Constant Units, 48 OFF Electrolysis Of Table Salt Element Electrolysis of molten sodium chloride. Gases may be formed at the electrodes. Salts are ionic compounds which can be electrolysed when they are in the molten state. Electrolysis involves using electricity to break down electrolytes to form elements. Cacl 2, hydrogen gas is produced at the cathode, not the metallic. There are two important points to make about these two. Electrolysis Of Table Salt Element.
From studyrocket.co.uk
Electrolysis GCSE Chemistry Science) OCR Revision Study Rocket Electrolysis Of Table Salt Element Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Electrolysis of aqueous salts •during electrolysis of the aqueous salt, e.g. Electrolysis of molten sodium chloride. The products of electrolysis can be predicted for a given. Electrolysis involves using electricity to break down electrolytes to form elements. Gases may be formed at the electrodes. Cacl 2,. Electrolysis Of Table Salt Element.
From sphweb.bumc.bu.edu
Chemical Elements Atoms Electrolysis Of Table Salt Element In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Electrolysis of aqueous salts •during electrolysis of the aqueous salt, e.g. Cacl 2, hydrogen gas is produced at the cathode, not the metallic. Gases may be formed at the electrodes. Electrolysis of an aqueous solution of table salt (nacl,. Electrolysis Of Table Salt Element.
From www.vecteezy.com
Electrolysis of Sodium Chloride solution 18891988 Vector Art at Vecteezy Electrolysis Of Table Salt Element Gases may be formed at the electrodes. Electrolysis involves using electricity to break down electrolytes to form elements. There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series.. Electrolysis Of Table Salt Element.
From periodictable.me
Sodium Valence Electrons Sodium Valency (Na) with Dot Diagram Electrolysis Of Table Salt Element Cacl 2, hydrogen gas is produced at the cathode, not the metallic. Gases may be formed at the electrodes. Salts are ionic compounds which can be electrolysed when they are in the molten state. There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. Electrolysis involves using electricity to. Electrolysis Of Table Salt Element.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrolysis Of Table Salt Element Electrolysis involves using electricity to break down electrolytes to form elements. There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Cacl 2, hydrogen gas is produced at the cathode, not the metallic. Salts are. Electrolysis Of Table Salt Element.
From ijmmm.ustb.edu.cn
Recovery and separation of rare earth elements by molten salt electrolysis Electrolysis Of Table Salt Element There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. Salts are ionic compounds which can be electrolysed when they are in the molten state. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Electrolysis of molten sodium chloride. Electrolysis of aqueous salts •during. Electrolysis Of Table Salt Element.
From chemrevisionigcse.blogspot.com
IGCSE Chemistry Electrolysis Electrolysis Of Table Salt Element Cacl 2, hydrogen gas is produced at the cathode, not the metallic. Salts are ionic compounds which can be electrolysed when they are in the molten state. The products of electrolysis can be predicted for a given. Gases may be formed at the electrodes. Electrolysis involves using electricity to break down electrolytes to form elements. Molten (liquid) sodium chloride can. Electrolysis Of Table Salt Element.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Electrolysis Of Table Salt Element The products of electrolysis can be predicted for a given. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Cacl 2, hydrogen gas is produced at the cathode, not the metallic. Salts are ionic compounds which can be electrolysed when they are in the molten state. Electrolysis involves using electricity to break down electrolytes to. Electrolysis Of Table Salt Element.
From www.youtube.com
Electrolysis of Salt Solutions YouTube Electrolysis Of Table Salt Element In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. Electrolysis involves using electricity to break down electrolytes to form elements. Cacl 2, hydrogen gas. Electrolysis Of Table Salt Element.
From www.nagwa.com
Question Video Electrolysis of Molten Sodium Salts Nagwa Electrolysis Of Table Salt Element There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Electrolysis involves using electricity. Electrolysis Of Table Salt Element.
From mungfali.com
Electrolysis Of Sodium Chloride Diagram Electrolysis Of Table Salt Element There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. The products of electrolysis can be predicted for a given. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. Electrolysis of aqueous salts •during electrolysis of the aqueous salt, e.g. Gases may be formed. Electrolysis Of Table Salt Element.
From www.freepik.com
Free Vector Electrolysis diagram experiment for education Electrolysis Of Table Salt Element Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. Cacl 2, hydrogen gas is produced at the cathode, not the metallic. Electrolysis of aqueous salts •during electrolysis of the aqueous salt, e.g. In this explainer, we will learn how to predict the products of electrolysis. Electrolysis Of Table Salt Element.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolysis Of Table Salt Element Electrolysis of molten sodium chloride. Gases may be formed at the electrodes. Electrolysis of aqueous salts •during electrolysis of the aqueous salt, e.g. There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. Salts are ionic compounds which can be electrolysed when they are in the molten state. Electrolysis. Electrolysis Of Table Salt Element.
From www.youtube.com
Electrolysis of Salt Solution at Home in Under 1 minute to produce Hydrogen and Chlorine YouTube Electrolysis Of Table Salt Element Cacl 2, hydrogen gas is produced at the cathode, not the metallic. Salts are ionic compounds which can be electrolysed when they are in the molten state. Electrolysis involves using electricity to break down electrolytes to form elements. Molten (liquid) sodium chloride can be electrolyzed to produce sodium metal and chlorine gas. There are two important points to make about. Electrolysis Of Table Salt Element.
From www.tes.com
Electrolysis of Salt Solutions GCSE Lesson (SC10b CC9b) Teaching Resources Electrolysis Of Table Salt Element In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Electrolysis of an aqueous solution of table salt (nacl, or sodium choride) produces aqueous sodium hydroxide and chlorine, although usually only in minute amounts. There are two important points to make about these two commercial processes and about the. Electrolysis Of Table Salt Element.
From brainly.in
The diagram below shows electrolysis of sodium chloride solution. True or false? Product C is Electrolysis Of Table Salt Element Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis of molten sodium chloride. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Salts are ionic compounds which can be electrolysed when they are in the molten state. Gases may be formed at the electrodes. There. Electrolysis Of Table Salt Element.
From www.slideserve.com
PPT 21 Electrochemistry PowerPoint Presentation, free download ID826545 Electrolysis Of Table Salt Element Electrolysis involves using electricity to break down electrolytes to form elements. In this explainer, we will learn how to predict the products of electrolysis of aqueous salt solutions using the reactivity series. Salts are ionic compounds which can be electrolysed when they are in the molten state. The products of electrolysis can be predicted for a given. Electrolysis of aqueous. Electrolysis Of Table Salt Element.
From chemnotcheem.com
Electrolysis guidebook O Level Chemistry Notes Electrolysis Of Table Salt Element Gases may be formed at the electrodes. There are two important points to make about these two commercial processes and about the electrolysis of molten salts in general. The products of electrolysis can be predicted for a given. Electrolysis of aqueous salts •during electrolysis of the aqueous salt, e.g. Cacl 2, hydrogen gas is produced at the cathode, not the. Electrolysis Of Table Salt Element.