How The Electric Pressure Cooker Works . Upon receiving this heat energy, the bonds holding the water molecules together begin to break. When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to boil and turn into vapor. As the pressure inside the cooker increases, the boiling point of water also rises. a pressure cooker works on a simple principle: Normally, water boils at 100°c (212°f) at sea level. you need to apply energy in the form of heat to boil water. Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. A sealed pot, with a lot of steam inside, builds up. Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). but the single biggest difference is this:
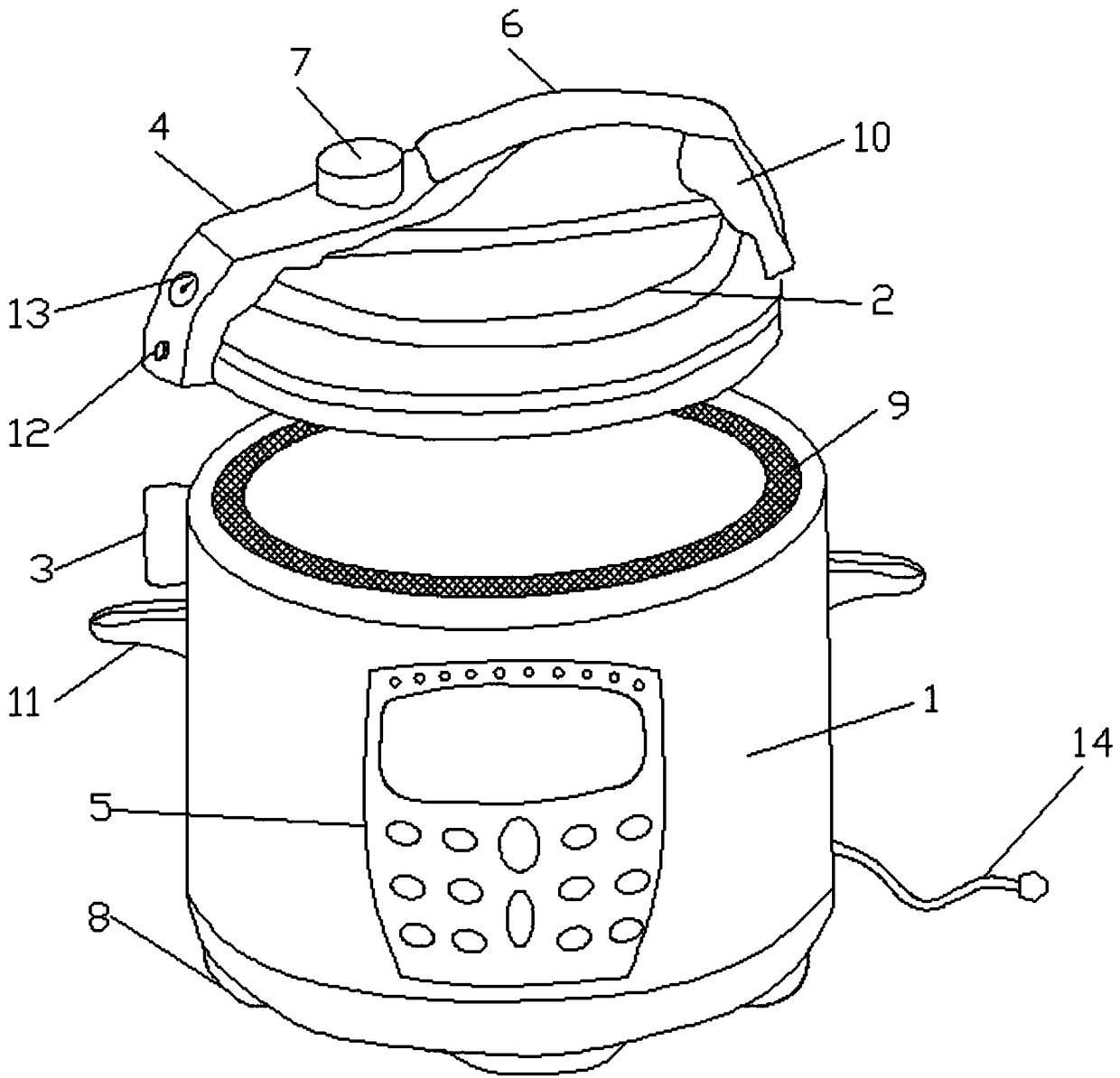
from eureka.patsnap.com
a pressure cooker works on a simple principle: Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. A sealed pot, with a lot of steam inside, builds up. Upon receiving this heat energy, the bonds holding the water molecules together begin to break. but the single biggest difference is this: you need to apply energy in the form of heat to boil water. As the pressure inside the cooker increases, the boiling point of water also rises. Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). Normally, water boils at 100°c (212°f) at sea level. When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to boil and turn into vapor.
Electric pressure cooker vapor exhausting device Eureka Patsnap
How The Electric Pressure Cooker Works but the single biggest difference is this: a pressure cooker works on a simple principle: you need to apply energy in the form of heat to boil water. A sealed pot, with a lot of steam inside, builds up. Normally, water boils at 100°c (212°f) at sea level. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. As the pressure inside the cooker increases, the boiling point of water also rises. Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). but the single biggest difference is this: Upon receiving this heat energy, the bonds holding the water molecules together begin to break. When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to boil and turn into vapor. Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model.
From storables.com
9 Superior Electric Pressure Rice Cooker For 2024 Storables How The Electric Pressure Cooker Works Normally, water boils at 100°c (212°f) at sea level. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. As the pressure inside the cooker increases, the boiling point of water also rises. When the internal vapor pressure equals the pressure exerted on it. How The Electric Pressure Cooker Works.
From robots.net
How To Use The Electric Pressure Cooker How The Electric Pressure Cooker Works Normally, water boils at 100°c (212°f) at sea level. A sealed pot, with a lot of steam inside, builds up. Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to boil and turn. How The Electric Pressure Cooker Works.
From storables.com
How Much Time Is Needed To Add For Electric Pressure Cooker Vs Stovetop How The Electric Pressure Cooker Works you need to apply energy in the form of heat to boil water. Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. but the single biggest difference is this: A sealed pot, with a lot of steam inside, builds up. As the pressure inside the cooker increases, the boiling. How The Electric Pressure Cooker Works.
From www.johnlewis.com
Instant Duo 6 7in1 MultiUse Electric Pressure Cooker, 5.7L How The Electric Pressure Cooker Works a pressure cooker works on a simple principle: but the single biggest difference is this: Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). you need to apply energy in the form of heat to boil water. When the internal vapor pressure equals the pressure exerted on it by the atmosphere,. How The Electric Pressure Cooker Works.
From www.vectorstock.com
How does a pressure cooker work and prepares food Vector Image How The Electric Pressure Cooker Works a pressure cooker works on a simple principle: Upon receiving this heat energy, the bonds holding the water molecules together begin to break. you need to apply energy in the form of heat to boil water. As the pressure inside the cooker increases, the boiling point of water also rises. When the internal vapor pressure equals the pressure. How The Electric Pressure Cooker Works.
From storables.com
How Does An Electric Pressure Cooker Work Storables How The Electric Pressure Cooker Works When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to boil and turn into vapor. Normally, water boils at 100°c (212°f) at sea level. A sealed pot, with a lot of steam inside, builds up. but the single biggest difference is this: Once again, lower pressure means lower temperature, so. How The Electric Pressure Cooker Works.
From farmfoodfamily.com
A Detailed Guide on How to Use an Electric Pressure Cooker How The Electric Pressure Cooker Works A sealed pot, with a lot of steam inside, builds up. Normally, water boils at 100°c (212°f) at sea level. As the pressure inside the cooker increases, the boiling point of water also rises. a pressure cooker works on a simple principle: the key scientific principle at play in pressure cooking is the direct relationship between pressure and. How The Electric Pressure Cooker Works.
From loegdboem.blob.core.windows.net
Instant Pot Pressure Cooker How Does It Work at Stephanie Rose blog How The Electric Pressure Cooker Works you need to apply energy in the form of heat to boil water. A sealed pot, with a lot of steam inside, builds up. Normally, water boils at 100°c (212°f) at sea level. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics.. How The Electric Pressure Cooker Works.
From www.youtube.com
5 Meals To Make In The Electric Pressure Cooker YouTube How The Electric Pressure Cooker Works the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). you need to apply energy in the form of heat to boil water. Upon receiving this heat energy,. How The Electric Pressure Cooker Works.
From www.canarymedia.com
How do induction stoves actually work? Canary Media How The Electric Pressure Cooker Works the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. a pressure cooker works on a simple principle: Normally, water boils at 100°c (212°f) at sea level. Once again, lower pressure means lower temperature, so cooking times will be longer when using an. How The Electric Pressure Cooker Works.
From robots.net
How To Use My Electric Pressure Cooker As A Slow Cooker How The Electric Pressure Cooker Works you need to apply energy in the form of heat to boil water. When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to boil and turn into vapor. Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). a pressure cooker works on a. How The Electric Pressure Cooker Works.
From reviewroller.in
5 Best (Affordable) Electric Pressure Cookers Cooks Fast How The Electric Pressure Cooker Works A sealed pot, with a lot of steam inside, builds up. but the single biggest difference is this: Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. Normally, water boils at 100°c (212°f) at sea level. a pressure cooker works on a simple principle: the key scientific principle. How The Electric Pressure Cooker Works.
From eureka.patsnap.com
Electric pressure cooker vapor exhausting device Eureka Patsnap How The Electric Pressure Cooker Works but the single biggest difference is this: Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). A sealed pot, with a lot of steam inside, builds up. you need to apply energy in the form of heat to boil water. the key scientific principle at play in pressure cooking is the. How The Electric Pressure Cooker Works.
From ovenspot.com
How do Pressure Cookers Work? Pressure Cooking 101 How The Electric Pressure Cooker Works As the pressure inside the cooker increases, the boiling point of water also rises. Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. Upon receiving this heat energy, the bonds holding the water molecules together begin. How The Electric Pressure Cooker Works.
From www.youtube.com
How does the Pressure cooker works? YouTube How The Electric Pressure Cooker Works Upon receiving this heat energy, the bonds holding the water molecules together begin to break. Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). a pressure cooker works on a simple principle: As the pressure inside the cooker increases, the boiling point of water also rises. the key scientific principle at play. How The Electric Pressure Cooker Works.
From www.thespruceeats.com
How Do Pressure Cookers Work? The Science Behind Them How The Electric Pressure Cooker Works Normally, water boils at 100°c (212°f) at sea level. but the single biggest difference is this: you need to apply energy in the form of heat to boil water. When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to boil and turn into vapor. A sealed pot, with a. How The Electric Pressure Cooker Works.
From fyodbkuts.blob.core.windows.net
How Does Pressure Cooker Work On Instant Pot at Tonya Smith blog How The Electric Pressure Cooker Works Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. but the single biggest difference is this: A sealed pot, with a lot of steam inside, builds up. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas. How The Electric Pressure Cooker Works.
From farmfoodfamily.com
A Detailed Guide on How to Use an Electric Pressure Cooker How The Electric Pressure Cooker Works Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). you need to apply energy in the form of heat to boil water. Normally, water boils at 100°c (212°f) at sea level. As the pressure inside the cooker increases, the boiling point of water also rises. Once again, lower pressure means lower temperature, so. How The Electric Pressure Cooker Works.
From www.hippressurecooking.com
The difference between stove top and electric pressure cookers ⋆ hip How The Electric Pressure Cooker Works When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to boil and turn into vapor. Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). you need to apply energy in the form of heat to boil water. but the single biggest difference is. How The Electric Pressure Cooker Works.
From www.tffn.net
How Does a Pressure Cooker Work? Exploring the Physics and Benefits How The Electric Pressure Cooker Works a pressure cooker works on a simple principle: Upon receiving this heat energy, the bonds holding the water molecules together begin to break. A sealed pot, with a lot of steam inside, builds up. Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. Electric pressure cookers operate at lower pressure. How The Electric Pressure Cooker Works.
From medium.com
How to use the Electric Pressure Cooker correctly? by How The Electric Pressure Cooker Works Normally, water boils at 100°c (212°f) at sea level. Upon receiving this heat energy, the bonds holding the water molecules together begin to break. you need to apply energy in the form of heat to boil water. a pressure cooker works on a simple principle: Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts. How The Electric Pressure Cooker Works.
From storables.com
How Do You Release Pressure From An Electric Pressure Cooker Storables How The Electric Pressure Cooker Works A sealed pot, with a lot of steam inside, builds up. Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. Normally, water boils at 100°c (212°f) at sea level. but the single biggest difference is this: you need to apply energy in the form of heat to boil water.. How The Electric Pressure Cooker Works.
From exyfjutfv.blob.core.windows.net
Does A Pressure Cooker Make Noise When Cooking at Teresa Majeski blog How The Electric Pressure Cooker Works the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). Normally, water boils at 100°c (212°f) at sea level. A sealed pot, with a lot of steam inside, builds. How The Electric Pressure Cooker Works.
From www.youtube.com
Get to know basics about your Electric Pressure Cooker YouTube How The Electric Pressure Cooker Works a pressure cooker works on a simple principle: Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). Normally, water boils at 100°c (212°f) at sea level. but the single biggest difference is this: When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to. How The Electric Pressure Cooker Works.
From www.pinterest.com
Pin on Instant pot How The Electric Pressure Cooker Works Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. Normally, water boils at 100°c (212°f) at sea level. A sealed pot, with a lot of steam inside, builds up. When the internal vapor pressure equals the. How The Electric Pressure Cooker Works.
From storables.com
Which Is The Best Electric Pressure Cooker? Storables How The Electric Pressure Cooker Works As the pressure inside the cooker increases, the boiling point of water also rises. you need to apply energy in the form of heat to boil water. Normally, water boils at 100°c (212°f) at sea level. a pressure cooker works on a simple principle: Once again, lower pressure means lower temperature, so cooking times will be longer when. How The Electric Pressure Cooker Works.
From www.vrogue.co
How Does A Pressure Cooker Work Miss Vickie vrogue.co How The Electric Pressure Cooker Works As the pressure inside the cooker increases, the boiling point of water also rises. A sealed pot, with a lot of steam inside, builds up. Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). a pressure cooker works on a simple principle: you need to apply energy in the form of heat. How The Electric Pressure Cooker Works.
From robots.net
How to Fix an Electric Pressure Cooker How The Electric Pressure Cooker Works Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. Upon receiving this heat energy, the bonds holding the water molecules together begin to break. you need to apply energy in the form of heat to boil water. the key scientific principle at play in pressure cooking is the direct. How The Electric Pressure Cooker Works.
From www.alamy.com
This stock photo depicts a visually appealing diagram illustrating the How The Electric Pressure Cooker Works A sealed pot, with a lot of steam inside, builds up. but the single biggest difference is this: Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. When the internal vapor pressure equals the pressure. How The Electric Pressure Cooker Works.
From home.howstuffworks.com
1 Timer and Delayed Start Features 5 Features to Look for When You How The Electric Pressure Cooker Works but the single biggest difference is this: Normally, water boils at 100°c (212°f) at sea level. Upon receiving this heat energy, the bonds holding the water molecules together begin to break. When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to boil and turn into vapor. a pressure cooker. How The Electric Pressure Cooker Works.
From www.youtube.com
Top 6 Best Electric Pressure Cookers 2024! Your Shortcut to Gourmet How The Electric Pressure Cooker Works Once again, lower pressure means lower temperature, so cooking times will be longer when using an electric model. you need to apply energy in the form of heat to boil water. When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to boil and turn into vapor. As the pressure inside. How The Electric Pressure Cooker Works.
From www.pressurecookrecipes.com
How to Use a Pressure Cooker Simple Guide by Amy + Jacky How The Electric Pressure Cooker Works Normally, water boils at 100°c (212°f) at sea level. you need to apply energy in the form of heat to boil water. As the pressure inside the cooker increases, the boiling point of water also rises. When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to boil and turn into. How The Electric Pressure Cooker Works.
From www.youtube.com
Best Electric Pressure Cookers 2023 Which is the 1 best Cooker? YouTube How The Electric Pressure Cooker Works A sealed pot, with a lot of steam inside, builds up. When the internal vapor pressure equals the pressure exerted on it by the atmosphere, the water will start to boil and turn into vapor. a pressure cooker works on a simple principle: As the pressure inside the cooker increases, the boiling point of water also rises. but. How The Electric Pressure Cooker Works.
From www.chaffinluhana.com
Pressure Cooker Explosion and Burn Lawyers (Live Chat 24/7) How The Electric Pressure Cooker Works Electric pressure cookers operate at lower pressure (12 psi) than their stovetop counterparts (15 psi). a pressure cooker works on a simple principle: Upon receiving this heat energy, the bonds holding the water molecules together begin to break. A sealed pot, with a lot of steam inside, builds up. Once again, lower pressure means lower temperature, so cooking times. How The Electric Pressure Cooker Works.
From www.easyanddelish.com
How does a Pressure Cooker Work? (Guide & Recipes) Easy and Delish How The Electric Pressure Cooker Works you need to apply energy in the form of heat to boil water. Upon receiving this heat energy, the bonds holding the water molecules together begin to break. a pressure cooker works on a simple principle: Normally, water boils at 100°c (212°f) at sea level. the key scientific principle at play in pressure cooking is the direct. How The Electric Pressure Cooker Works.