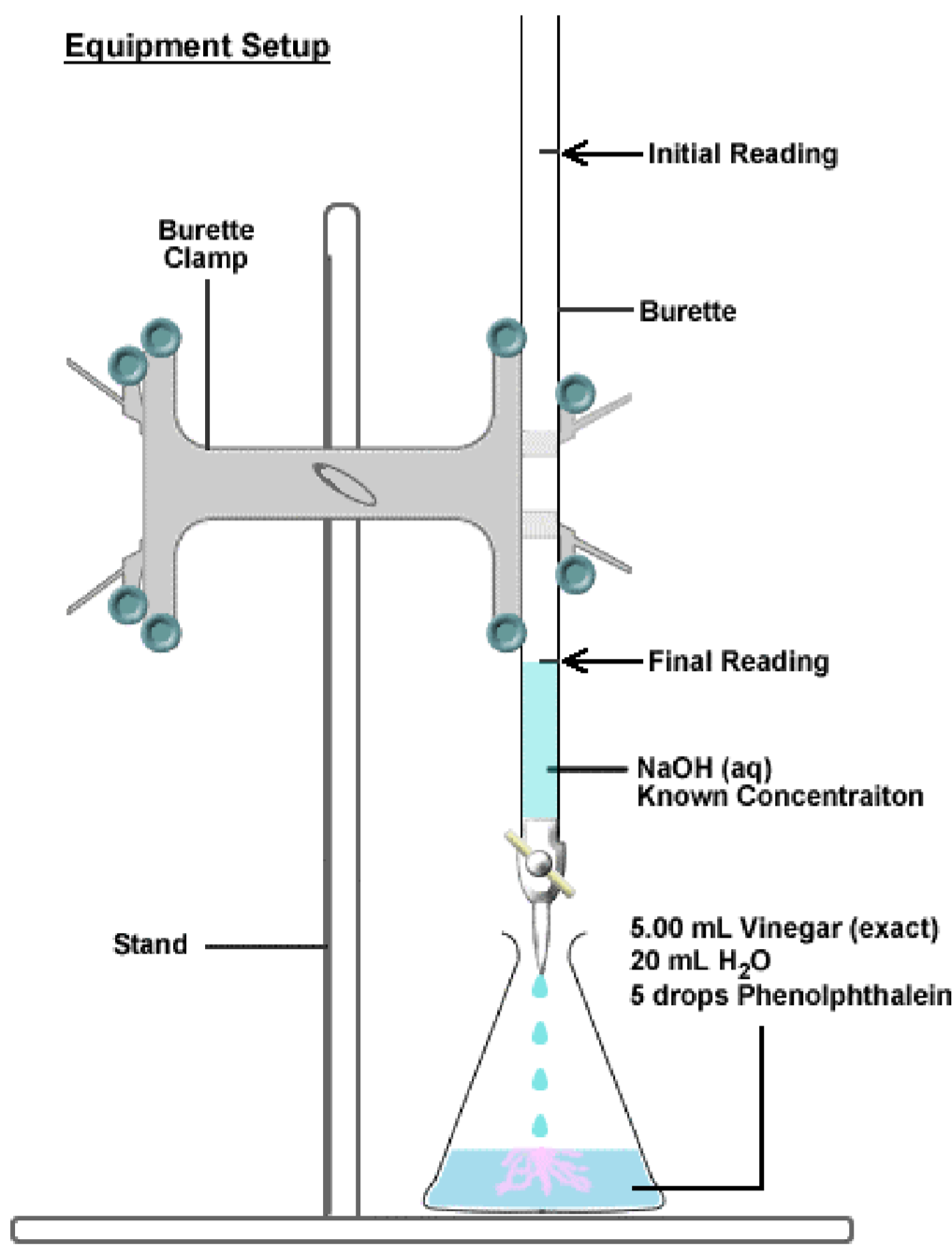
Titration Practical Equipment . 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. How to prepare for titration. Make up a volumetric solution and carry out a simple. A titration is a very commonly used type of quantitative analysis. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. It is based on accurately measured volumes of chemicals. conduct practical investigations to analyse the concentration of an unknown acid or base by titration; use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the.
from chem.libretexts.org
How to prepare for titration. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. conduct practical investigations to analyse the concentration of an unknown acid or base by titration; revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. It is based on accurately measured volumes of chemicals. Make up a volumetric solution and carry out a simple. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. A titration is a very commonly used type of quantitative analysis. Includes kit list and safety. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with.
11 Titration of Vinegar (Experiment) Chemistry LibreTexts
Titration Practical Equipment conduct practical investigations to analyse the concentration of an unknown acid or base by titration; It is based on accurately measured volumes of chemicals. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. conduct practical investigations to analyse the concentration of an unknown acid or base by titration; How to prepare for titration. A titration is a very commonly used type of quantitative analysis. Make up a volumetric solution and carry out a simple. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with.
From chem.libretexts.org
4.8 Stoichiometry of Reactions in Solution Titrations Chemistry Titration Practical Equipment Includes kit list and safety. It is based on accurately measured volumes of chemicals. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. How to prepare for titration. A titration. Titration Practical Equipment.
From en.ppt-online.org
Titration and AcidBase Neutralization online presentation Titration Practical Equipment revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. It is based on accurately measured volumes of chemicals. Make up a volumetric solution and carry out a simple. Includes kit list and safety. How to prepare for titration. use this class practical to explore titration, producing the. Titration Practical Equipment.
From gioxhprmf.blob.core.windows.net
Titration Significant Figures at Darlene Maloy blog Titration Practical Equipment A titration is a very commonly used type of quantitative analysis. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. Includes kit list and safety. How to prepare for titration. It is based on accurately measured volumes of chemicals. 1.1 a titration involves measuring the exact volume. Titration Practical Equipment.
From www.revisechemistry.uk
Monitoring chemical reactions (chemistry only) OCR Gateway C5 Titration Practical Equipment use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Includes kit list and safety. Make up a volumetric solution and carry out a simple. A titration is a very commonly used type. Titration Practical Equipment.
From gioldvmyt.blob.core.windows.net
Titration Chemical Method at Karl Twiggs blog Titration Practical Equipment revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. How to prepare for titration. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. determination of the reacting volumes of solutions of a strong acid. Titration Practical Equipment.
From www.vrogue.co
Titration Practice Worksheet Worksheets For All Free vrogue.co Titration Practical Equipment revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. How to prepare for titration. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. conduct practical investigations to analyse the concentration of an unknown acid or base by titration;. Titration Practical Equipment.
From stock.adobe.com
Acid base titration experiment and phases of color change during Titration Practical Equipment Make up a volumetric solution and carry out a simple. It is based on accurately measured volumes of chemicals. Includes kit list and safety. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry. Titration Practical Equipment.
From giofzfwwv.blob.core.windows.net
Acid Base Titration To Determine The Concentration at Latonya John blog Titration Practical Equipment 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Make. Titration Practical Equipment.
From www.etsy.com
Titration Equipment Set Complete Single Buret Burete Assembly Etsy Canada Titration Practical Equipment It is based on accurately measured volumes of chemicals. conduct practical investigations to analyse the concentration of an unknown acid or base by titration; determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Includes kit list and safety. A titration is a very commonly used type of quantitative analysis. How. Titration Practical Equipment.
From ubicaciondepersonas.cdmx.gob.mx
Titration Equipment Kit ubicaciondepersonas.cdmx.gob.mx Titration Practical Equipment conduct practical investigations to analyse the concentration of an unknown acid or base by titration; revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. determination of the. Titration Practical Equipment.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Titration Practical Equipment 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. Make up a volumetric solution and carry out a simple. A titration is a very commonly used type of quantitative analysis. determination of the reacting volumes of solutions of a strong acid and a strong alkali by. Titration Practical Equipment.
From gioxbplkr.blob.core.windows.net
Titration Equipment at Jose Baker blog Titration Practical Equipment revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. Make up a volumetric solution and carry out a simple. How to prepare for titration. It is based on accurately measured volumes of chemicals. use this class practical to explore titration, producing the salt sodium chloride with sodium. Titration Practical Equipment.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Practical Equipment use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety. conduct practical investigations to analyse the concentration of an unknown acid or base by titration; determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. How to. Titration Practical Equipment.
From www.savemyexams.com
Required Practical Strong Acid & Strong Alkali Titration AQA GCSE Titration Practical Equipment How to prepare for titration. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. Make up a volumetric solution and carry out a simple. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. use this class practical to. Titration Practical Equipment.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Titration Practical Equipment determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Includes kit list and safety. A titration is a very commonly used type of quantitative analysis. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. conduct practical investigations. Titration Practical Equipment.
From www.homesciencetools.com
Titration Kit Titration Lab Equipment for Chemistry HST Titration Practical Equipment A titration is a very commonly used type of quantitative analysis. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. Make up a volumetric solution and carry out a. Titration Practical Equipment.
From gioceaglx.blob.core.windows.net
Titration Name Of Apparatus at Darrell West blog Titration Practical Equipment conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Make up a volumetric solution and carry out a simple. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. Includes kit list and safety. It is based on accurately measured volumes of. Titration Practical Equipment.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Practical Equipment How to prepare for titration. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Make up a volumetric solution and carry out a simple. Includes kit list and safety. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. A. Titration Practical Equipment.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Titration Practical Equipment Includes kit list and safety. conduct practical investigations to analyse the concentration of an unknown acid or base by titration; A titration is a very commonly used type of quantitative analysis. How to prepare for titration. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. Make up. Titration Practical Equipment.
From www.sciencephoto.com
Equipment used in a titration Stock Image C035/9302 Science Photo Titration Practical Equipment revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. Make up a volumetric solution and carry out a simple. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. Includes kit list and safety. It is. Titration Practical Equipment.
From exymjkekq.blob.core.windows.net
Titration In Chemistry Equipment at Barbara Adamson blog Titration Practical Equipment A titration is a very commonly used type of quantitative analysis. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. conduct practical investigations to analyse the concentration of. Titration Practical Equipment.
From gioaymxup.blob.core.windows.net
Equipment For Titration Experiment at Gloria McFarland blog Titration Practical Equipment revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. conduct practical investigations to analyse the concentration of an unknown acid or base by titration; How. Titration Practical Equipment.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources Titration Practical Equipment It is based on accurately measured volumes of chemicals. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. Includes kit list and safety. 1.1 a titration involves measuring. Titration Practical Equipment.
From fyoapfhcs.blob.core.windows.net
Titration Lab Simple at Justin Funk blog Titration Practical Equipment Includes kit list and safety. conduct practical investigations to analyse the concentration of an unknown acid or base by titration; How to prepare for titration. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that. Titration Practical Equipment.
From quizlet.com
Titration and making a standard solution Required practical 1 Diagram Titration Practical Equipment It is based on accurately measured volumes of chemicals. conduct practical investigations to analyse the concentration of an unknown acid or base by titration; use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. A titration is a very commonly used type of quantitative analysis. How to prepare for titration.. Titration Practical Equipment.
From fyospissj.blob.core.windows.net
Titration Equipment Diagram at Patricia Pendergrast blog Titration Practical Equipment 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. A titration is a very commonly used type of quantitative analysis. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. How to prepare for titration. revision notes on. Titration Practical Equipment.
From gioaymxup.blob.core.windows.net
Equipment For Titration Experiment at Gloria McFarland blog Titration Practical Equipment A titration is a very commonly used type of quantitative analysis. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. How to prepare for titration. . Titration Practical Equipment.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Practical Equipment A titration is a very commonly used type of quantitative analysis. Make up a volumetric solution and carry out a simple. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. conduct practical investigations to analyse the concentration of an unknown acid or base by titration; It is. Titration Practical Equipment.
From www.homesciencetools.com
Titration Kit Titration Lab Equipment for Chemistry HST Titration Practical Equipment A titration is a very commonly used type of quantitative analysis. conduct practical investigations to analyse the concentration of an unknown acid or base by titration; use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. 1.1 a titration involves measuring the exact volume of a reagent solution (the. Titration Practical Equipment.
From giooyspjv.blob.core.windows.net
Titration Lab Level 2 at Kevin Fields blog Titration Practical Equipment revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. How to prepare for titration. Includes kit list and safety. A titration is a very commonly used. Titration Practical Equipment.
From www.vrogue.co
Acid Base Titration Equipment vrogue.co Titration Practical Equipment 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. conduct practical investigations to analyse the concentration of an unknown acid or base by titration; revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. It. Titration Practical Equipment.
From giorsvmpe.blob.core.windows.net
Titration Clipart at Antonio Jacobs blog Titration Practical Equipment It is based on accurately measured volumes of chemicals. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. A titration is a very commonly used type of quantitative analysis. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Make. Titration Practical Equipment.
From www.zogirls.com
Performing a Titration & Volumetric Analysis (4.1.2) AQA A Level Titration Practical Equipment use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. How to. Titration Practical Equipment.
From ar.inspiredpencil.com
Acid Base Titration Equipment Titration Practical Equipment determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Includes kit list and safety. A titration is a very commonly used type of quantitative analysis. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. conduct practical investigations to analyse the. Titration Practical Equipment.
From mungfali.com
Acid Base Titration Procedure Titration Practical Equipment Includes kit list and safety. revision notes on 4.1.2 performing a titration & volumetric analysis for the aqa a level chemistry syllabus, written by the. determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. A titration is a very commonly used type of quantitative analysis. It is based on accurately. Titration Practical Equipment.