Discussion For Titration Of Naoh And Hcl . A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly added to 50.0 ml of 0.10 m hcl, the ph. Once the tip of the burette is full of. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Hcl + naoh → nacl + h 2 o. Hydrochloric acid reacts with sodium hydroxide. According to the reaction equation. Titrate with naoh solution till the first color change. This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh).
from www.numerade.com
Hcl + naoh → nacl + h 2 o. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. According to the reaction equation. Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly added to 50.0 ml of 0.10 m hcl, the ph. Once the tip of the burette is full of. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh). Hydrochloric acid reacts with sodium hydroxide. Titrate with naoh solution till the first color change.
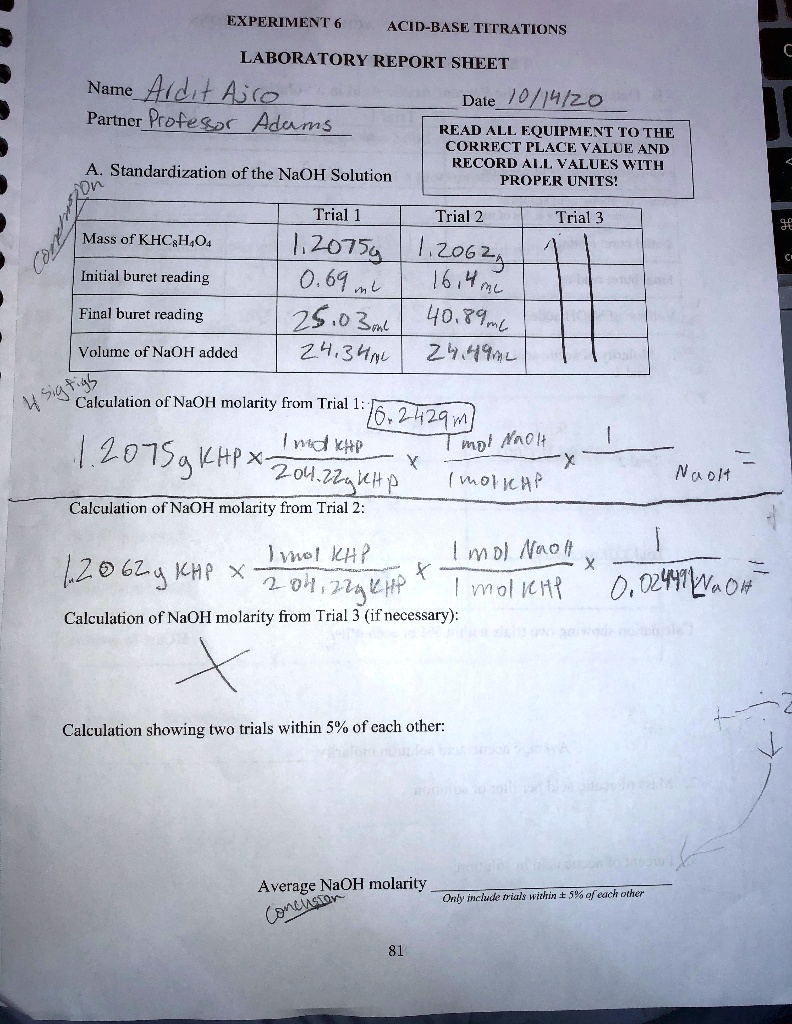
SOLVED Text EXPERIMENT 6 ACIDBASE TITRATIONS LABORATORY REPORT SHEET
Discussion For Titration Of Naoh And Hcl Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly added to 50.0 ml of 0.10 m hcl, the ph. This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh). Hcl + naoh → nacl + h 2 o. Titrate with naoh solution till the first color change. According to the reaction equation. Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly added to 50.0 ml of 0.10 m hcl, the ph. Once the tip of the burette is full of. Hydrochloric acid reacts with sodium hydroxide. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution.
From mungfali.com
HCl NaOH Titration Discussion For Titration Of Naoh And Hcl Once the tip of the burette is full of. According to the reaction equation. Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. Titrate with naoh solution till the first color change. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is,. Discussion For Titration Of Naoh And Hcl.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Discussion For Titration Of Naoh And Hcl Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly added to 50.0 ml of 0.10 m hcl, the ph. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Discussion For Titration Of Naoh And Hcl.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Discussion For Titration Of Naoh And Hcl Hydrochloric acid reacts with sodium hydroxide. Once the tip of the burette is full of. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Titrate with naoh solution till the first color change. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it. Discussion For Titration Of Naoh And Hcl.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Discussion For Titration Of Naoh And Hcl Hcl + naoh → nacl + h 2 o. According to the reaction equation. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Titrate with naoh solution till the first color change. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\). Discussion For Titration Of Naoh And Hcl.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Discussion For Titration Of Naoh And Hcl Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. Hcl + naoh → nacl + h 2 o. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Once the tip of the. Discussion For Titration Of Naoh And Hcl.
From studylib.net
Experiment (1) Standardization of sodium hydroxide NaOH solution Discussion For Titration Of Naoh And Hcl Hydrochloric acid reacts with sodium hydroxide. This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh). Titrate with naoh solution till the first color change. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Once the tip. Discussion For Titration Of Naoh And Hcl.
From mungfali.com
Acid Base Titration Lab Discussion For Titration Of Naoh And Hcl Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly added to 50.0 ml of 0.10 m hcl, the ph. According to the reaction equation. Hydrochloric acid reacts with sodium hydroxide. Using a small funnel, pour a few cubic centimetres of. Discussion For Titration Of Naoh And Hcl.
From www.scribd.com
Standardization of Hydrochloric Acid Titration Hydrochloric Acid Discussion For Titration Of Naoh And Hcl According to the reaction equation. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Hcl + naoh → nacl + h 2 o. Once the tip of the burette. Discussion For Titration Of Naoh And Hcl.
From mungfali.com
HCl NaOH Titration Discussion For Titration Of Naoh And Hcl Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Once the tip of the burette is full of. Titrate with naoh solution till the first color change. Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker. Discussion For Titration Of Naoh And Hcl.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Discussion For Titration Of Naoh And Hcl According to the reaction equation. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. Hydrochloric acid reacts with sodium. Discussion For Titration Of Naoh And Hcl.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Discussion For Titration Of Naoh And Hcl Hcl + naoh → nacl + h 2 o. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Once the tip of the burette is full of. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. According to. Discussion For Titration Of Naoh And Hcl.
From giolmzfsh.blob.core.windows.net
Titration Of Acid And Base Lab Report Introduction at Andrew Alvarado blog Discussion For Titration Of Naoh And Hcl Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly added to 50.0 ml of 0.10 m hcl, the ph. Using a small. Discussion For Titration Of Naoh And Hcl.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Discussion For Titration Of Naoh And Hcl This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh). Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. According to the reaction equation. Once the tip. Discussion For Titration Of Naoh And Hcl.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Discussion For Titration Of Naoh And Hcl Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly added to 50.0 ml of 0.10 m hcl, the ph. According to the reaction equation. Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the. Discussion For Titration Of Naoh And Hcl.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Discussion For Titration Of Naoh And Hcl A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly added to 50.0 ml of 0.10 m hcl, the. Discussion For Titration Of Naoh And Hcl.
From www.youtube.com
Titration of an unknown acid with a standardized Sodium Hydroxide Discussion For Titration Of Naoh And Hcl This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh). According to the reaction equation. Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly added to. Discussion For Titration Of Naoh And Hcl.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Discussion For Titration Of Naoh And Hcl Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. Once the tip of the burette is full of. Hydrochloric acid reacts with sodium hydroxide. Hcl + naoh → nacl + h 2 o. This leaves the final product to simply be. Discussion For Titration Of Naoh And Hcl.
From www.sliderbase.com
Titration Discussion For Titration Of Naoh And Hcl Hydrochloric acid reacts with sodium hydroxide. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. Titrate with naoh solution. Discussion For Titration Of Naoh And Hcl.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Discussion For Titration Of Naoh And Hcl This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh). Titrate with naoh solution till the first color change. Hcl + naoh → nacl + h 2 o. Hydrochloric acid reacts with sodium hydroxide. A titration is an analytical procedure used to determine the accurate concentration. Discussion For Titration Of Naoh And Hcl.
From hxetahfoe.blob.core.windows.net
Analysis Titration at Pauline Estill blog Discussion For Titration Of Naoh And Hcl According to the reaction equation. Hydrochloric acid reacts with sodium hydroxide. Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and. Discussion For Titration Of Naoh And Hcl.
From lessonlibraryemersed.z13.web.core.windows.net
Titration Practical Questions And Answers Discussion For Titration Of Naoh And Hcl This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh). Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. According to the reaction equation. Hydrochloric acid reacts with sodium hydroxide. A titration is an analytical procedure used. Discussion For Titration Of Naoh And Hcl.
From www.studocu.com
Discussion titration 2 Discussion Volume initial de NaOH Considérant Discussion For Titration Of Naoh And Hcl Once the tip of the burette is full of. Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly added to 50.0 ml of 0.10 m hcl, the ph. Titrate with naoh solution till the first color change. Using a small. Discussion For Titration Of Naoh And Hcl.
From www.chegg.com
Lab 8 Analysis of Vinegar by Titration This page Discussion For Titration Of Naoh And Hcl Once the tip of the burette is full of. Titrate with naoh solution till the first color change. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Hydrochloric acid reacts with sodium hydroxide. According to the reaction equation. Hcl + naoh → nacl + h 2 o. Using a small funnel,. Discussion For Titration Of Naoh And Hcl.
From www.numerade.com
SOLVED Text EXPERIMENT 6 ACIDBASE TITRATIONS LABORATORY REPORT SHEET Discussion For Titration Of Naoh And Hcl A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\). Discussion For Titration Of Naoh And Hcl.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Discussion For Titration Of Naoh And Hcl Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly added to 50.0 ml of 0.10 m hcl, the ph. Titrate with naoh solution till the first color change. Hydrochloric acid reacts with sodium hydroxide. Once the tip of the burette. Discussion For Titration Of Naoh And Hcl.
From ar.inspiredpencil.com
Titration Setup Diagram Discussion For Titration Of Naoh And Hcl Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh). Figure 16.5.2 the titration of (a) a strong. Discussion For Titration Of Naoh And Hcl.
From www.chegg.com
Solved Week 1 Oxalic AcidSodium Hydroxide Titration Mass Discussion For Titration Of Naoh And Hcl Once the tip of the burette is full of. This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh). Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Titrate with naoh solution till the first color change.. Discussion For Titration Of Naoh And Hcl.
From hxeedchjz.blob.core.windows.net
Discussion Of Titration at Effie Hope blog Discussion For Titration Of Naoh And Hcl Hcl + naoh → nacl + h 2 o. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Figure 16.5.2 the titration of (a) a strong acid with a strong base and (b) a strong base with a strong acid (a) as 0.20 m naoh is slowly. Discussion For Titration Of Naoh And Hcl.
From www.youtube.com
Titration Of NaOH with Oxalic Acid ( Class XI, Practical1) YouTube Discussion For Titration Of Naoh And Hcl Once the tip of the burette is full of. Hydrochloric acid reacts with sodium hydroxide. This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh). A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard. Discussion For Titration Of Naoh And Hcl.
From www.youtube.com
Titration of HCl with NaOH YouTube Discussion For Titration Of Naoh And Hcl Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. Once the tip of the burette is full of. Hcl + naoh → nacl. Discussion For Titration Of Naoh And Hcl.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Discussion For Titration Of Naoh And Hcl Hcl + naoh → nacl + h 2 o. According to the reaction equation. Once the tip of the burette is full of. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. This leaves the final product to simply be water, this is displayed in the following. Discussion For Titration Of Naoh And Hcl.
From mungfali.com
HCl NaOH Titration Discussion For Titration Of Naoh And Hcl According to the reaction equation. Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. Once the tip of the burette is full of. Hcl + naoh → nacl + h 2 o. This leaves the final product to simply be water,. Discussion For Titration Of Naoh And Hcl.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Discussion For Titration Of Naoh And Hcl Titrate with naoh solution till the first color change. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and \(\ce{naoh}\) is the titrant. Hcl + naoh → nacl + h 2 o. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Once the. Discussion For Titration Of Naoh And Hcl.
From www.hotzxgirl.com
Titration Procedure Pdf Hot Sex Picture Discussion For Titration Of Naoh And Hcl Once the tip of the burette is full of. This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh). According to the reaction equation. Hcl + naoh → nacl + h 2 o. A titration is an analytical procedure used to determine the accurate concentration of. Discussion For Titration Of Naoh And Hcl.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Discussion For Titration Of Naoh And Hcl This leaves the final product to simply be water, this is displayed in the following example involving hydrochloric acid (hcl) and sodium hydroxide (naoh). Using a small funnel, pour a few cubic centimetres of 0.4 m hydrochloric acid into the burette, with the tap open and a beaker under the open tap. A titration is an analytical procedure used to. Discussion For Titration Of Naoh And Hcl.