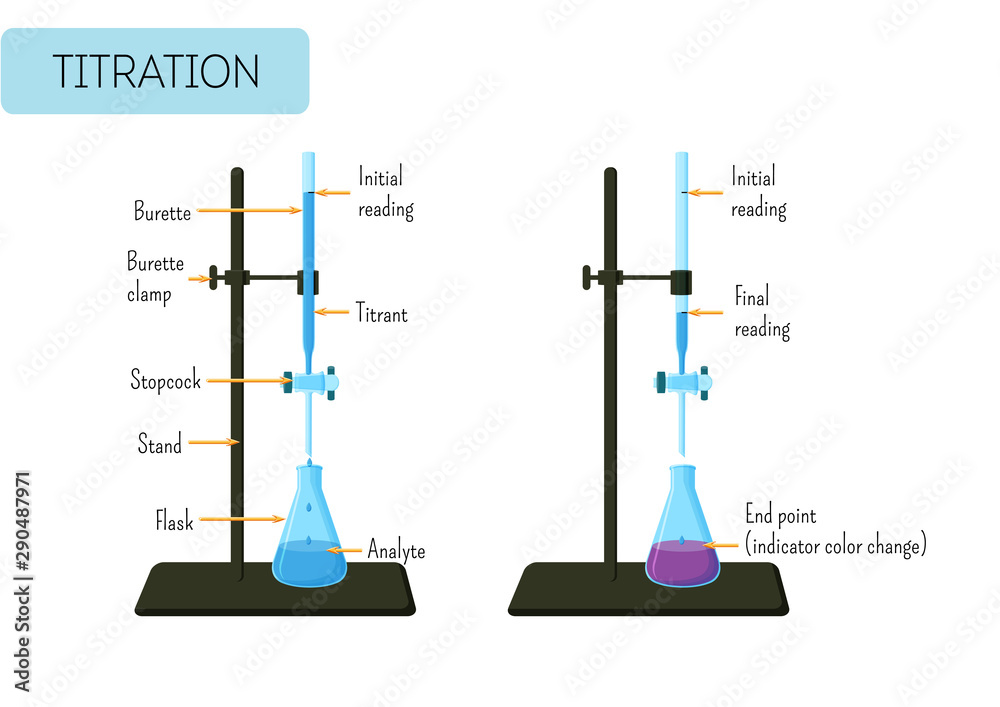
Flask After Titration . In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely: As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the.
from stock.adobe.com
Using the initial and final reading, the volume added can be determined quite precisely: After the titration has reached the endpoint, a final volume is read from the buret. If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the.
Laboratory experiment of acid base titration with glass burette and
Flask After Titration If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the. After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely: In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Flask After Titration After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely: As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration. Flask After Titration.
From stock.adobe.com
step of titration of acid and base or standardisation using Flask After Titration After the titration has reached the endpoint, a final volume is read from the buret. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. If solution a is titrated against solution b, it means that solution a is. Flask After Titration.
From stock.adobe.com
Laboratory experiment of acid base titration with glass burette and Flask After Titration If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself. After the. Flask After Titration.
From www.slideteam.net
Experiment Sample Icon With Titration Flask Presentation PowerPoint Flask After Titration As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the. If you were to. Flask After Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Flask After Titration In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the. Using the initial and final. Flask After Titration.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Flask After Titration If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. Using the initial and final reading, the volume added can be determined quite precisely: As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical. Flask After Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Flask After Titration Using the initial and final reading, the volume added can be determined quite precisely: If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. After the titration has reached the endpoint, a final volume is read from the buret. As long as the pipette. Flask After Titration.
From www.indiamart.com
Titration Flask at best price in Ambala by S.K.APPLIANCES ID 17536517462 Flask After Titration If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself. In this. Flask After Titration.
From www.differencebetween.com
What is the Difference Between Erlenmeyer Flask and Florence Flask Flask After Titration After the titration has reached the endpoint, a final volume is read from the buret. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. As long as the pipette is rinsed appropriately and the correct quantity of the. Flask After Titration.
From cartoondealer.com
The Erlenmeyer Flask On Bench Laboratory, With Bright Pink Solvent Flask After Titration After the titration has reached the endpoint, a final volume is read from the buret. If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the. Flask After Titration.
From www.gettyimages.com
Burette And Flask For Titration In Chemical Laboratory HighRes Stock Flask After Titration If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself. After the. Flask After Titration.
From childhealthpolicy.vumc.org
Vitamin c determination by iodine titration lab report. Vitamin C Flask After Titration If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. As long as the pipette. Flask After Titration.
From www.alamy.com
Titration, titrimetry or volumetric analysis. A burette and Erlenmeyer Flask After Titration In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the. As long as the pipette. Flask After Titration.
From www.daigger.com
Pyrex Glass Titration Flasks Flask After Titration Using the initial and final reading, the volume added can be determined quite precisely: After the titration has reached the endpoint, a final volume is read from the buret. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed.. Flask After Titration.
From www.amkglass.com
500mL Titration Flask, ASTM D3242 Flask After Titration If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the. After the titration has reached the endpoint, a final volume is read from the buret. As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask,. Flask After Titration.
From www.alamy.com
Titration experiment. Student using a burette and conical flask to Flask After Titration Using the initial and final reading, the volume added can be determined quite precisely: In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. If solution a is titrated against solution b, it means that solution a is in. Flask After Titration.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Flask After Titration If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. Using the initial and final reading, the volume added can be determined quite precisely: If solution a is titrated against solution b, it means that solution a is in the conical flask and solution. Flask After Titration.
From www.vrogue.co
Top 10 Apparatus For Titration Of 2020 No Place Calle vrogue.co Flask After Titration Using the initial and final reading, the volume added can be determined quite precisely: If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution. Flask After Titration.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Flask After Titration If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. After the titration has reached the endpoint, a final volume is read from the buret. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical. Flask After Titration.
From www.nagwa.com
Question Video Explaining Why the Erlenmeyer Flask Must Be Swirled Flask After Titration Using the initial and final reading, the volume added can be determined quite precisely: In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. As long as the pipette is rinsed appropriately and the correct quantity of the solution. Flask After Titration.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Flask After Titration After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely: If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the. If you were to calculate the concentration. Flask After Titration.
From www.slideserve.com
PPT titrations PowerPoint Presentation, free download ID3456377 Flask After Titration Using the initial and final reading, the volume added can be determined quite precisely: As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself. If solution a is titrated against solution b, it means that solution a is. Flask After Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Flask After Titration As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself. After the titration has reached the endpoint, a final volume is read from the buret. If solution a is titrated against solution b, it means that solution a. Flask After Titration.
From www.chegg.com
Solved Which flask below. A or B. represents an accurate Flask After Titration After the titration has reached the endpoint, a final volume is read from the buret. As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself. In this tutorial, you will find information on titration, including the chemicals that. Flask After Titration.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Flask After Titration After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely: As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration. Flask After Titration.
From www.fishersci.ca
DWK Life Sciences Kimble™ KIMAX™ Titration Flasks Fisher Scientific Flask After Titration As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the. If you were to. Flask After Titration.
From www.alamy.com
Titration flask hires stock photography and images Alamy Flask After Titration As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself. If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. Using the. Flask After Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Flask After Titration After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely: If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the. In this tutorial, you will find information. Flask After Titration.
From saylordotorg.github.io
Solution Concentrations Flask After Titration If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. If you were to calculate. Flask After Titration.
From www.chem-rg.com
Used Kimble 26500250 KIMAX Titration Flask 250mL for Sale at Flask After Titration After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely: If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. In this tutorial, you will. Flask After Titration.
From www.pixazsexy.com
Sticker Featuring An Erlenmeyer Flask Conical Flask Titration Flask Flask After Titration As long as the pipette is rinsed appropriately and the correct quantity of the solution is transferred into the conical flask, its concentration in the flask will not affect the titration itself. Using the initial and final reading, the volume added can be determined quite precisely: After the titration has reached the endpoint, a final volume is read from the. Flask After Titration.
From www.vrogue.co
Acid Base Titration With Phenolphthalein Indicator Wo vrogue.co Flask After Titration If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the. Using the initial and final reading, the volume added can. Flask After Titration.
From www.dreamstime.com
The Erlenmeyer Flask on Bench Laboratory, with Bright Pink Solvent Flask After Titration If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely: As long as the pipette. Flask After Titration.
From www.indiamart.com
JKI Borosilicate Glass 250 Ml Laboratory Titration Flask, Rs 290 /piece Flask After Titration If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. As long as. Flask After Titration.
From scienceready.com.au
Titration Standard Solution, Washing, Setup HSC Chemistry Science Flask After Titration In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. If you were to calculate the concentration of acid from the titration, and you add distilled water to the erlenmeyer flask, then yes, you would affect. Using the initial. Flask After Titration.