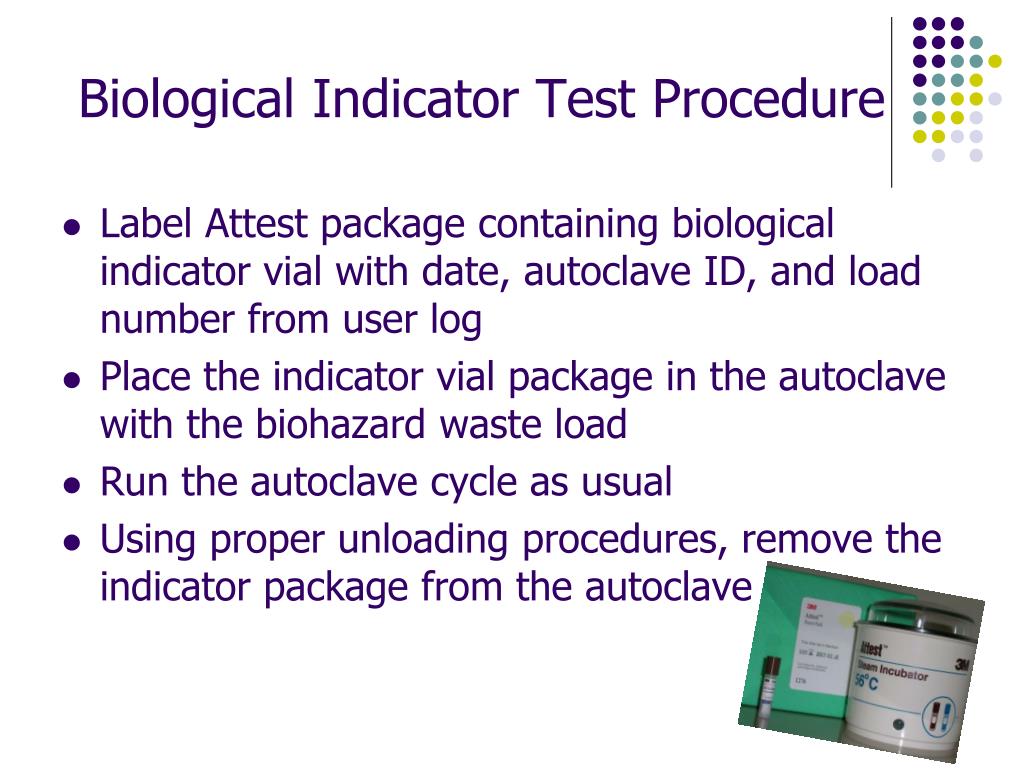
Biological Indicator Testing Procedure . Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and.
from www.slideserve.com
Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and.
PPT Autoclave Training PowerPoint Presentation, free download ID
Biological Indicator Testing Procedure Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality.
From daiichibiotech.com.my
Biological Indicators Daiichi Biotech Service Biological Indicator Testing Procedure Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. This sop is applicable for. Biological Indicator Testing Procedure.
From rrsmed.ir
Steam Biological Indicator 10^5Roshan Rai SepahanRRS Sterilization Test Biological Indicator Testing Procedure This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization.. Biological Indicator Testing Procedure.
From gkeaustralia.com
SelfContained Biological Indicators for Sterilisation Monitoring Biological Indicator Testing Procedure Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators (bis) are. Biological Indicator Testing Procedure.
From www.slideserve.com
PPT Autoclave Training PowerPoint Presentation, free download ID Biological Indicator Testing Procedure Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Biological. Biological Indicator Testing Procedure.
From www.studocu.com
Sterilization indicators 1 Biological indicator (Spore Test) Test Biological Indicator Testing Procedure Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators have. Biological Indicator Testing Procedure.
From rrsmed.ir
Plasma Biological IndicatorRoshan Rai SepahanRRS Sterilization Test Biological Indicator Testing Procedure Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. This sop is applicable. Biological Indicator Testing Procedure.
From emechmedical.com
Biological Indicator Spore Test BT20 Emech Medical New Zealand Biological Indicator Testing Procedure Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and.. Biological Indicator Testing Procedure.
From www.fisaude.eu
Biological Indicator EZTest (25 units) Fisaude Store Biological Indicator Testing Procedure Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. This. Biological Indicator Testing Procedure.
From mumuplug.weebly.com
Biological indicator spore testing procedure mumuplug Biological Indicator Testing Procedure Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators are. Biological Indicator Testing Procedure.
From www.slideserve.com
PPT Autoclave Training PowerPoint Presentation, free download ID Biological Indicator Testing Procedure Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. This sop is applicable. Biological Indicator Testing Procedure.
From decisionsindentistry.com
Role of Chemical and Biological Indicators in Infection Prevention Biological Indicator Testing Procedure This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization. Biological Indicator Testing Procedure.
From www.slideserve.com
PPT Autoclave Training PowerPoint Presentation ID1247416 Biological Indicator Testing Procedure Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators (bis) are. Biological Indicator Testing Procedure.
From dimensionsofdentalhygiene.com
Ensure Infection Control Compliance With Chemical and Biological Biological Indicator Testing Procedure Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization). Biological Indicator Testing Procedure.
From pharmasciences.in
Types of biological indicators PharmaSciences Biological Indicator Testing Procedure Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. This. Biological Indicator Testing Procedure.
From www.youtube.com
Biological Indicator of Sterilization Overview of Sterilization Biological Indicator Testing Procedure Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization). Biological Indicator Testing Procedure.
From www.omnia-health.com
Biological Indicators ECS Srl Biological Indicator Testing Procedure Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Biological indicators are utilized. Biological Indicator Testing Procedure.
From www.indiamart.com
Biological Indicators Steam Eto at Rs 5000/packet Biological Biological Indicator Testing Procedure Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. This. Biological Indicator Testing Procedure.
From dimensionsofdentalhygiene.com
Ensure Infection Control Compliance With Chemical and Biological Biological Indicator Testing Procedure Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Provide. Biological Indicator Testing Procedure.
From www.proppermfg.com
Biological Indicator Test DuoSpore® Biological Indicator Testing Procedure This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators are utilized. Biological Indicator Testing Procedure.
From www.slideserve.com
PPT Autoclave Training PowerPoint Presentation, free download ID Biological Indicator Testing Procedure Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial.. Biological Indicator Testing Procedure.
From www.sychem.co.uk
Why Use Biological Indicators? Sychem Biological Indicator Testing Procedure Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators are. Biological Indicator Testing Procedure.
From www.slideserve.com
PPT Monitoring the Sterilization Process PowerPoint Presentation ID Biological Indicator Testing Procedure This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators have. Biological Indicator Testing Procedure.
From www.youtube.com
How to Activate Biological Indicator YouTube Biological Indicator Testing Procedure Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Provide. Biological Indicator Testing Procedure.
From www.slideserve.com
PPT Monitoring the Sterilization Process PowerPoint Presentation Biological Indicator Testing Procedure Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization). Biological Indicator Testing Procedure.
From dokumen.tips
(PDF) Biological Indicator Testing Procedures Ver 1 Biosafety Program Biological Indicator Testing Procedure Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Provide. Biological Indicator Testing Procedure.
From pharmablog.in
Receiving, Issuance and Verification of Biological IndicatorsSOP Biological Indicator Testing Procedure Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Biological. Biological Indicator Testing Procedure.
From www.youtube.com
In Office Biological Monitoring How to use Crosstex ConFirm® Incubators Biological Indicator Testing Procedure This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators are utilized. Biological Indicator Testing Procedure.
From ntep.in
Quality Control Process of the Autoclave Knowledge Base Biological Indicator Testing Procedure Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Biological indicators (bis). Biological Indicator Testing Procedure.
From www.sychem.co.uk
Why Use Biological Indicators? Sychem Biological Indicator Testing Procedure Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators are designed. Biological Indicator Testing Procedure.
From www.slideserve.com
PPT Basics of Sterilization PowerPoint Presentation, free download Biological Indicator Testing Procedure This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization.. Biological Indicator Testing Procedure.
From metrolab.blog
Biological Indicator Selection Metrolab Blog Biological Indicator Testing Procedure Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators are utilized. Biological Indicator Testing Procedure.
From skan.com
SKAN BI® SKAN Biological Indicator Testing Procedure Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Provide. Biological Indicator Testing Procedure.
From studylib.net
Biological Indicator Spore Testing Procedure Biological Indicator Testing Procedure This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained.. Biological Indicator Testing Procedure.
From www.honeymanlaboratories.com
Biological Indicator BI Testing Biological Indicator Testing Procedure This sop is applicable for the testing of biological indicators (bi) on the receipt (spore population verification and organism characterization) and. Provide guidance for using biological indicators for steam, hydrogen peroxide plasma and ethylene oxide (eto) sterilization. Biological indicators have evolved over the past 50 years and continue to provide the only direct measure of sterilization lethality. Biological indicators are. Biological Indicator Testing Procedure.
From www.slideserve.com
PPT Steam sterilization theory and equipment PowerPoint Presentation Biological Indicator Testing Procedure Biological indicators are utilized to test the effectiveness of a given sterilization process and the equipment used, by assessing microbial. Biological indicators are designed to show by the survival of test microorganisms whether specified sterilization conditions have been attained. Biological indicators (bis) are used and tested in support of sterilization validations and routine product release for ethylene oxide sterilization. Provide. Biological Indicator Testing Procedure.