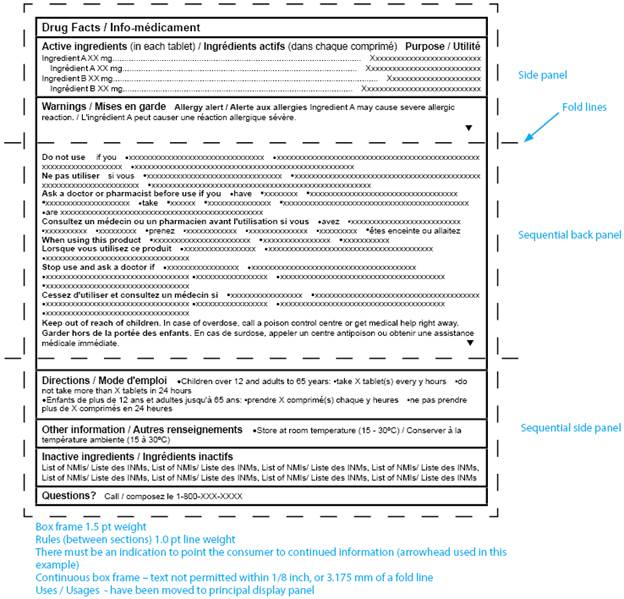
Drug Substance Labeling Requirements . All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer a wide range of stakeholder queries which relate to the. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. There are also changes to the eu’s approach to labelling of imps that in addition to changing the approach to providing expiry. The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make it possible to ascertain— (a).
from www.canada.ca
This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make it possible to ascertain— (a). From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer a wide range of stakeholder queries which relate to the. There are also changes to the eu’s approach to labelling of imps that in addition to changing the approach to providing expiry. All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best.
Labelling requirements for nonprescription drugs guidance document
Drug Substance Labeling Requirements This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. There are also changes to the eu’s approach to labelling of imps that in addition to changing the approach to providing expiry. Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer a wide range of stakeholder queries which relate to the. All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make it possible to ascertain— (a). This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk.
From www.icoptix.com
Labeling Laws/FDA and EU Guidance IC Optix Drug Substance Labeling Requirements This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the. Drug Substance Labeling Requirements.
From www.slideserve.com
PPT Overview of the New Content and Format Requirements for Drug Substance Labeling Requirements This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. There are also changes to the eu’s approach to labelling of imps that in addition to changing the approach to providing expiry. From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging. Drug Substance Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Drug Substance Labeling Requirements Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer a wide range of stakeholder queries which relate to the. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. From 1 january 2025, in order to enable medicines to use the same packaging and. Drug Substance Labeling Requirements.
From www.slideshare.net
Labeling of Drugs 21 CFR Part 201 Drug Substance Labeling Requirements Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer a wide range of stakeholder queries which relate to the. The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make it possible to ascertain— (a). All finished drug products should be identified. Drug Substance Labeling Requirements.
From www.fagronsterile.com
Controlled substances from a 503B outsourcing facility Drug Substance Labeling Requirements From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help. Drug Substance Labeling Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Drug Substance Labeling Requirements This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products. Drug Substance Labeling Requirements.
From www.grc-health.com
Investigational Medicinal Product labelling an overview — GRCHealth Drug Substance Labeling Requirements There are also changes to the eu’s approach to labelling of imps that in addition to changing the approach to providing expiry. Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer a wide range of stakeholder queries which relate to the. This document aims to set out uniform statements on storage conditions. Drug Substance Labeling Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Drug Substance Labeling Requirements From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer a wide range of stakeholder queries which relate to the. The ministers may by regulations require the use of certain. Drug Substance Labeling Requirements.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Drug Substance Labeling Requirements The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make it possible to ascertain— (a). Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer a wide range of stakeholder queries which relate to the. From 1 january 2025, in order to. Drug Substance Labeling Requirements.
From www.fda.gov
How Do I Use Prescription Drug Labeling FDA Drug Substance Labeling Requirements The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make it possible to ascertain— (a). From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. All finished drug products should be identified by labelling, as required. Drug Substance Labeling Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Drug Substance Labeling Requirements This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make it possible to ascertain— (a). All finished drug products should be identified by labelling, as required by the national legislation, bearing. Drug Substance Labeling Requirements.
From www.canada.ca
Labelling requirements for nonprescription drugs guidance document Drug Substance Labeling Requirements This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. There are also changes to the eu’s approach to labelling of imps that in addition to changing the approach to providing. Drug Substance Labeling Requirements.
From www.slideteam.net
Drug Substance Specification Pharmaceutical Development New Medicine Drug Substance Labeling Requirements This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer a wide. Drug Substance Labeling Requirements.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID1950891 Drug Substance Labeling Requirements From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help. Drug Substance Labeling Requirements.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Drug Substance Labeling Requirements This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make. Drug Substance Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Drug Substance Labeling Requirements There are also changes to the eu’s approach to labelling of imps that in addition to changing the approach to providing expiry. All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: From 1 january 2025, in order to enable medicines to use the same packaging and labelling across. Drug Substance Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Drug Substance Labeling Requirements This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best.. Drug Substance Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Drug Substance Labeling Requirements All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer a wide range of stakeholder queries which relate to the. From 1 january 2025, in order to enable medicines to use the. Drug Substance Labeling Requirements.
From www.slideserve.com
PPT FDA LABELING PowerPoint Presentation, free download ID3633953 Drug Substance Labeling Requirements This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer a wide range of stakeholder queries which relate to the. There are also changes to the eu’s approach to labelling of imps that in addition. Drug Substance Labeling Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Drug Substance Labeling Requirements This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. This guidance explains the legal framework for labelling and packaging as described in uk legislation and. Drug Substance Labeling Requirements.
From www.slideserve.com
PPT Drug and Product Labeling PowerPoint Presentation, free download Drug Substance Labeling Requirements From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. There are also changes to the eu’s approach to labelling of imps that in addition to. Drug Substance Labeling Requirements.
From www.slideserve.com
PPT Prescription Drug Labeling PowerPoint Presentation ID330259 Drug Substance Labeling Requirements This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make. Drug Substance Labeling Requirements.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID1950891 Drug Substance Labeling Requirements This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. There are also changes to the eu’s approach to labelling of imps that in addition to changing the approach to providing expiry. All finished drug products should be identified by labelling, as required by the national legislation, bearing at. Drug Substance Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Drug Substance Labeling Requirements All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the. Drug Substance Labeling Requirements.
From www.slideserve.com
PPT Drug and Product Labeling PowerPoint Presentation, free download Drug Substance Labeling Requirements The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make it possible to ascertain— (a). There are also changes to the eu’s approach to labelling of imps that in addition to changing the approach to providing expiry. From 1 january 2025, in order to enable medicines to use the. Drug Substance Labeling Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Drug Substance Labeling Requirements The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make it possible to ascertain— (a). From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. Gmp and gcp inspectors work closely with mhra clinical trials and. Drug Substance Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Drug Substance Labeling Requirements This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information:. Drug Substance Labeling Requirements.
From www.youtube.com
U.S. FDA Drug Labeling Requirements YouTube Drug Substance Labeling Requirements There are also changes to the eu’s approach to labelling of imps that in addition to changing the approach to providing expiry. The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make it possible to ascertain— (a). This document aims to set out uniform statements on storage conditions for. Drug Substance Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Drug Substance Labeling Requirements This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the. Drug Substance Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Drug Substance Labeling Requirements The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make it possible to ascertain— (a). This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives best. All finished drug products should be identified by labelling, as required by the national legislation, bearing. Drug Substance Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Drug Substance Labeling Requirements Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer a wide range of stakeholder queries which relate to the. All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: The ministers may by regulations require the use of certain forms of. Drug Substance Labeling Requirements.
From brennad-images.blogspot.com
Printable Prescription Warning Labels / Ers Solutions Pharmacy Drug Substance Labeling Requirements There are also changes to the eu’s approach to labelling of imps that in addition to changing the approach to providing expiry. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide support to help answer. Drug Substance Labeling Requirements.
From www.canada.ca
Industry requirements for nonprescription drug labels Canada.ca Drug Substance Labeling Requirements There are also changes to the eu’s approach to labelling of imps that in addition to changing the approach to providing expiry. This document aims to set out uniform statements on storage conditions for inclusion in the labelling of medicinal products and to. This guidance explains the legal framework for labelling and packaging as described in uk legislation and gives. Drug Substance Labeling Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Drug Substance Labeling Requirements The ministers may by regulations require the use of certain forms of labelling of a medicinal product in order to make it possible to ascertain— (a). All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: Gmp and gcp inspectors work closely with mhra clinical trials and regularly provide. Drug Substance Labeling Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Drug Substance Labeling Requirements All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information: From 1 january 2025, in order to enable medicines to use the same packaging and labelling across the uk, packaging for all uk. There are also changes to the eu’s approach to labelling of imps that in addition to. Drug Substance Labeling Requirements.