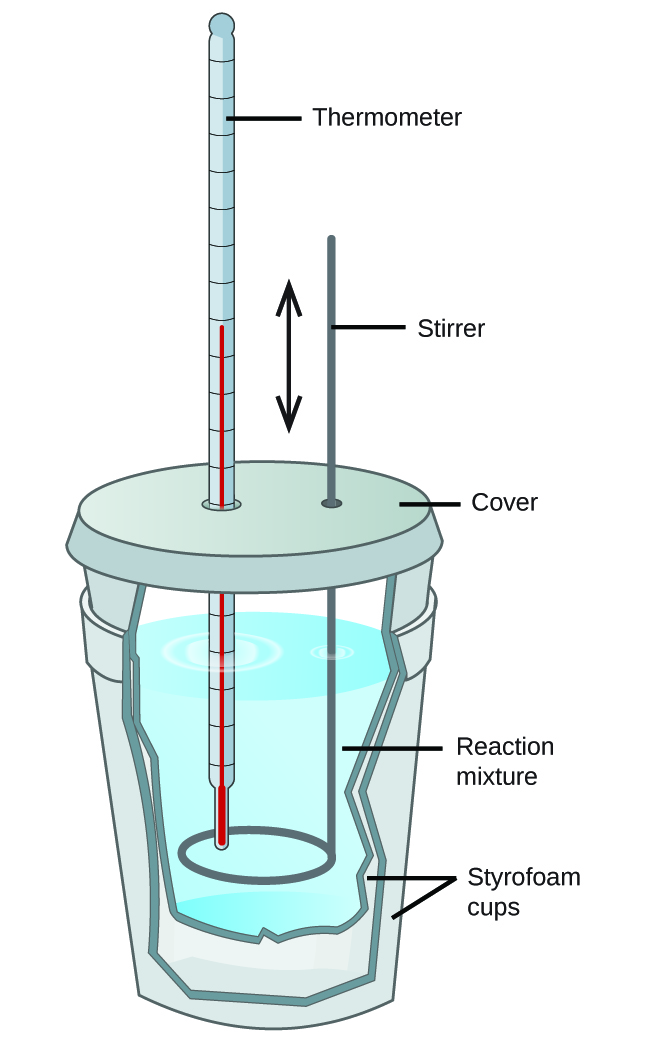
What Is Calorimeter . Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Technically, this is called an adiabatic. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Learn about different types of calorimeters, the calorimetry.
from
A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Technically, this is called an adiabatic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. Learn about different types of calorimeters, the calorimetry. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state.
What Is Calorimeter Technically, this is called an adiabatic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Learn about different types of calorimeters, the calorimetry. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. For example, when an exothermic. Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. Technically, this is called an adiabatic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction.
From
What Is Calorimeter Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Technically, this is called an adiabatic. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. What Is Calorimeter.
From
What Is Calorimeter A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example,. What Is Calorimeter.
From
What Is Calorimeter Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. For example, when an exothermic. Learn about different types of calorimeters, the calorimetry. Technically, this is called an adiabatic. A calorimeter is a device used to measure. What Is Calorimeter.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas What Is Calorimeter Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. Learn about different types of calorimeters, the calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings.. What Is Calorimeter.
From
What Is Calorimeter Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. Calorimeter, device for measuring the heat developed during a mechanical,. What Is Calorimeter.
From
What Is Calorimeter A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Technically, this is called an adiabatic. A calorimeter is a device that measures the heat involved in. What Is Calorimeter.
From
What Is Calorimeter Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Learn about different types of calorimeters, the calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic. A calorimeter is an instrument that has a thermally isolated. What Is Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Is Calorimeter Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. Learn about different types of calorimeters, the calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical. What Is Calorimeter.
From
What Is Calorimeter A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. Technically, this is called an adiabatic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Learn. What Is Calorimeter.
From
What Is Calorimeter Learn about different types of calorimeters, the calorimetry. Technically, this is called an adiabatic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn about the types, principle,. What Is Calorimeter.
From
What Is Calorimeter A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. Technically, this is called an adiabatic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. What Is Calorimeter.
From
What Is Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. Learn about. What Is Calorimeter.
From
What Is Calorimeter Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Technically, this is called an adiabatic. A calorimeter. What Is Calorimeter.
From www.3bscientific.com
Copper Calorimeter 1002659 U10366 Calorimeters 3B Scientific What Is Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. Learn about different types of calorimeters, the calorimetry. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is. What Is Calorimeter.
From
What Is Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn about the types, principle,. What Is Calorimeter.
From thermonine92.blogspot.com
Thermochemistry Calorimeter What Is Calorimeter A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is an instrument. What Is Calorimeter.
From
What Is Calorimeter Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state.. What Is Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 What Is Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn about different types of calorimeters, the calorimetry. A calorimeter is a device that measures the heat involved in a chemical. What Is Calorimeter.
From
What Is Calorimeter Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of. What Is Calorimeter.
From www.education.com
Calorimetry Bomb Calorimeter Experiment What Is Calorimeter A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to. What Is Calorimeter.
From www.wisegeek.com
What is Calorimetry? (with pictures) What Is Calorimeter Technically, this is called an adiabatic. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. For example, when an exothermic. Learn about different types of calorimeters, the calorimetry. Calorimeter, device for measuring the heat developed during a mechanical,. What Is Calorimeter.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter What Is Calorimeter A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Technically, this is called an adiabatic. Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry. What Is Calorimeter.
From
What Is Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. Learn about different types of calorimeters, the calorimetry. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the. What Is Calorimeter.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry What Is Calorimeter For example, when an exothermic. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating. What Is Calorimeter.
From
What Is Calorimeter Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Learn about different types of calorimeters, the calorimetry. A calorimeter is a device used to measure the amount of heat involved in. What Is Calorimeter.
From
What Is Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. Technically, this is called an adiabatic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. What Is Calorimeter.
From
What Is Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn about different types of calorimeters, the calorimetry. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Technically, this is called an adiabatic. Calorimetry is a field of thermochemistry. What Is Calorimeter.
From courses.lumenlearning.com
Calorimetry General Chemistry What Is Calorimeter Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn about different types of calorimeters, the calorimetry. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. For example, when an exothermic. Learn about the types, principle, instrumentation, and. What Is Calorimeter.
From saylordotorg.github.io
Calorimetry What Is Calorimeter Learn about different types of calorimeters, the calorimetry. Learn about the types, principle, instrumentation, and applications of calorimeters in chemistry. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state.. What Is Calorimeter.
From
What Is Calorimeter For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Learn about different types of calorimeters, the calorimetry. Learn about the types, principle, instrumentation,. What Is Calorimeter.
From byjus.com
The calorimeter is commonly made up of……..because it has……. What Is Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Technically, this is called an adiabatic. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. What Is Calorimeter.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide What Is Calorimeter Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Technically, this is called an adiabatic. Calorimeter, device for measuring the heat developed during a. What Is Calorimeter.
From www.3bscientific.com
Calorimeter with Heating Coil, 150 ml 1000822 U8441020 What Is Calorimeter For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Learn about different types of calorimeters, the calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Learn about the types, principle, instrumentation, and applications of calorimeters. What Is Calorimeter.
From
What Is Calorimeter Technically, this is called an adiabatic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. A calorimeter is a device used to measure the amount of heat involved in a chemical. What Is Calorimeter.
From
What Is Calorimeter For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device that measures the heat involved in a chemical reaction or physical change of state. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. What Is Calorimeter.