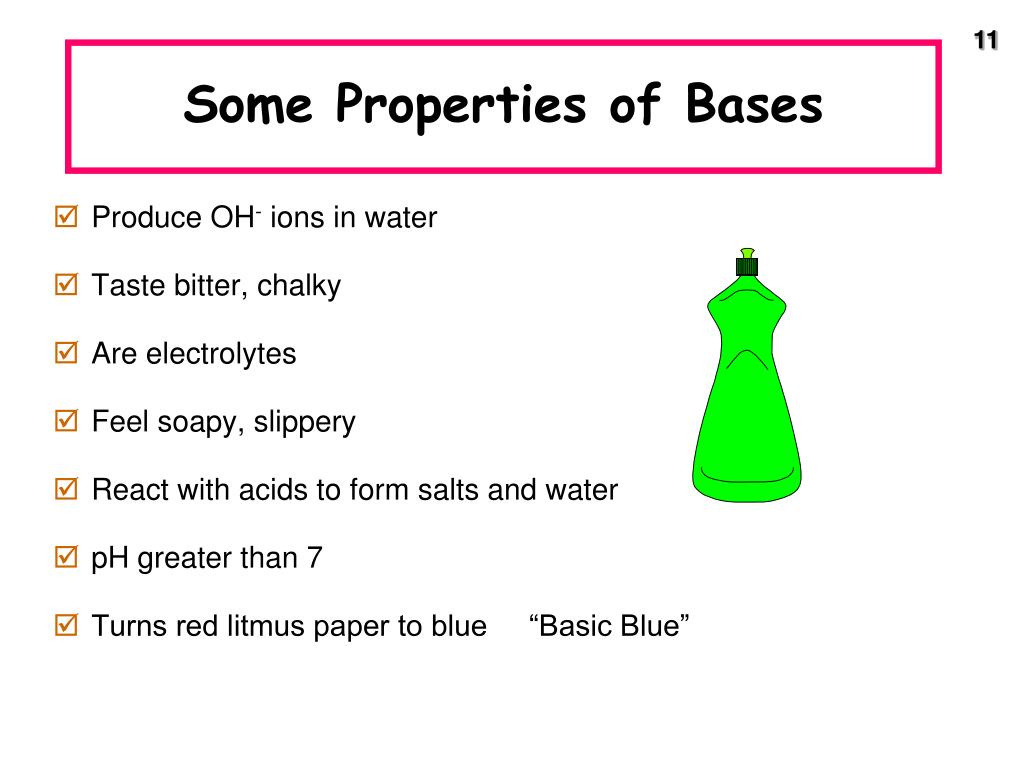
What Is The Three Properties Of A Base . Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Common bases include metal oxides, hydroxides or carbonates. Bases have properties that mostly contrast with those of acids. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. If a base dissolves in water, it is also referred to as an. Bases can be either strong or. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes.
from www.slideserve.com
Common bases include metal oxides, hydroxides or carbonates. Aqueous solutions of bases are also electrolytes. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Aqueous solutions of bases are also electrolytes. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Bases have properties that mostly contrast with those of acids. If a base dissolves in water, it is also referred to as an. Bases can be either strong or.
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free
What Is The Three Properties Of A Base Bases can be either strong or. Bases have properties that mostly contrast with those of acids. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Aqueous solutions of bases are also electrolytes. If a base dissolves in water, it is also referred to as an. Bases can be either strong or. Common bases include metal oxides, hydroxides or carbonates. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation, free download ID1994749 What Is The Three Properties Of A Base Common bases include metal oxides, hydroxides or carbonates. Bases can be either strong or. Aqueous solutions of bases are also electrolytes. Aqueous solutions of bases are also electrolytes. If a base dissolves in water, it is also referred to as an. Bases have properties that mostly contrast with those of acids. The bases are classified into three categories namely monoacidic. What Is The Three Properties Of A Base.
From www.teachoo.com
Bases and it's Properties (with Examples, Definition) Teachoo What Is The Three Properties Of A Base If a base dissolves in water, it is also referred to as an. Common bases include metal oxides, hydroxides or carbonates. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. Bases have properties that mostly contrast with those of acids. The bases are classified into three categories namely monoacidic base, diacidic base,. What Is The Three Properties Of A Base.
From animalia-life.club
Properties Of Bases What Is The Three Properties Of A Base Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. Bases have properties that mostly contrast with those of acids. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Bases have properties that mostly. What Is The Three Properties Of A Base.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry What Is The Three Properties Of A Base The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. In chemistry, a base is a substance that reacts with acids to. What Is The Three Properties Of A Base.
From animalia-life.club
Properties Of Bases What Is The Three Properties Of A Base Bases can be either strong or. Aqueous solutions of bases are also electrolytes. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Bases have properties that mostly contrast with those of acids. Common bases include metal oxides, hydroxides or carbonates. In chemistry, a base is a substance that. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5349215 What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. Common bases include metal oxides, hydroxides or carbonates. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Bases can be either strong or. Aqueous solutions of bases are also electrolytes. If a base dissolves in water, it is. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Acids, Bases, and Salts PowerPoint Presentation, free download What Is The Three Properties Of A Base Aqueous solutions of bases are also electrolytes. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. Bases have properties that mostly contrast with those of acids. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Acids, Bases, and Salts PowerPoint Presentation, free download What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. If a base dissolves in. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6539541 What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. Bases can. What Is The Three Properties Of A Base.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Aqueous solutions of bases are also electrolytes. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Bases can be either strong or. Bases have properties that mostly contrast with those of. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2789112 What Is The Three Properties Of A Base The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Bases can be either strong or. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Acid and Bases An Introduction PowerPoint Presentation, free What Is The Three Properties Of A Base If a base dissolves in water, it is also referred to as an. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Aqueous solutions. What Is The Three Properties Of A Base.
From www.youtube.com
Chemical properties of Bases Chemistry Class 10th Ch 10 YouTube What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. Bases have properties that mostly contrast with those of acids. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Aqueous solutions of bases are also electrolytes. Bases can be either strong or. Bases have properties that mostly contrast. What Is The Three Properties Of A Base.
From www.slideshare.net
Acids and bases ppt notes What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Aqueous solutions of bases are also electrolytes. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. If a base dissolves in water, it is. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Explaining the Properties of Acids & Bases PowerPoint What Is The Three Properties Of A Base Common bases include metal oxides, hydroxides or carbonates. Bases have properties that mostly contrast with those of acids. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Aqueous solutions of bases are also electrolytes. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Common bases include metal oxides, hydroxides or carbonates. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis. What Is The Three Properties Of A Base.
From hra.animalia-life.club
Properties Of Acids And Bases What Is The Three Properties Of A Base Aqueous solutions of bases are also electrolytes. Common bases include metal oxides, hydroxides or carbonates. Bases have properties that mostly contrast with those of acids. Bases have properties that mostly contrast with those of acids. Bases can be either strong or. If a base dissolves in water, it is also referred to as an. Aqueous solutions of bases are also. What Is The Three Properties Of A Base.
From slideplayer.com
Acids and Bases Operational definitions are based on observed What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. Bases can be either strong or. Bases have properties that mostly contrast with those of acids. Common bases include metal oxides, hydroxides or carbonates. Aqueous solutions of bases are also electrolytes. In chemistry,. What Is The Three Properties Of A Base.
From studylib.net
Properties of acids and bases What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. If a base dissolves in water, it is also referred to as an. The bases are classified into three categories namely monoacidic base, diacidic base, and. What Is The Three Properties Of A Base.
From animalia-life.club
Properties Of Bases What Is The Three Properties Of A Base Aqueous solutions of bases are also electrolytes. If a base dissolves in water, it is also referred to as an. Bases have properties that mostly contrast with those of acids. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Bases have properties that mostly contrast with those of. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Solutions, Acids, and Bases PowerPoint Presentation, free What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Chapter 8 PowerPoint Presentation, free download ID603230 What Is The Three Properties Of A Base Aqueous solutions of bases are also electrolytes. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Aqueous solutions of bases are also electrolytes. Common bases include metal oxides, hydroxides or carbonates. Aqueous solutions of bases are also electrolytes. In chemistry, a base is a substance that reacts with. What Is The Three Properties Of A Base.
From www.sliderbase.com
AcidBase Reactions Presentation Chemistry What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. Bases have properties that mostly contrast with those of acids. If a base dissolves in water, it is also referred to as an. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous. What Is The Three Properties Of A Base.
From www.youtube.com
3A 14.2 and 14.3 Acids & Bases Properties & Examples YouTube What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. If a base dissolves in water, it is also referred to as an. Bases have properties that mostly contrast with those of acids. Bases can be either strong or. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. In chemistry, a base. What Is The Three Properties Of A Base.
From animalia-life.club
Properties Of Bases What Is The Three Properties Of A Base Aqueous solutions of bases are also electrolytes. Bases can be either strong or. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Common bases include metal oxides, hydroxides or carbonates. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Bases. What Is The Three Properties Of A Base.
From animalia-life.club
Properties Of Bases What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. Bases have properties that mostly contrast with those of acids. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Aqueous solutions of bases are also electrolytes. In chemistry, a base is a substance that reacts with acids to. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Properties of Acids and Bases PowerPoint Presentation, free What Is The Three Properties Of A Base Aqueous solutions of bases are also electrolytes. Bases can be either strong or. If a base dissolves in water, it is also referred to as an. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. In chemistry, a base is a substance that reacts with acids to form a salt and which. What Is The Three Properties Of A Base.
From brainly.in
Give three chemical properties of base? Brainly.in What Is The Three Properties Of A Base Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. Common bases include metal oxides, hydroxides or carbonates. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Bases have properties that mostly contrast with those of acids. Bases have properties that. What Is The Three Properties Of A Base.
From animalia-life.club
Properties Of Bases What Is The Three Properties Of A Base Common bases include metal oxides, hydroxides or carbonates. Bases have properties that mostly contrast with those of acids. The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. If a base dissolves. What Is The Three Properties Of A Base.
From chemistry-examples-00.blogspot.com
70 CHEMISTRY EXAMPLES OF ACIDS AND BASES, BASES ACIDS EXAMPLES OF What Is The Three Properties Of A Base Bases can be either strong or. Bases have properties that mostly contrast with those of acids. If a base dissolves in water, it is also referred to as an. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Aqueous solutions of. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Properties of Acids and Bases PowerPoint Presentation, free What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Aqueous solutions of bases are also electrolytes. Common bases include metal oxides, hydroxides or carbonates. Bases have properties that mostly contrast with. What Is The Three Properties Of A Base.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry What Is The Three Properties Of A Base Bases have properties that mostly contrast with those of acids. Bases can be either strong or. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Acids and Bases Introduction PowerPoint Presentation, free What Is The Three Properties Of A Base In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Aqueous solutions of bases are also electrolytes. Bases have properties that mostly contrast with those of acids. If a base dissolves in water, it is also referred to as an. Aqueous solutions. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT Unit 3 PowerPoint Presentation, free download ID4697485 What Is The Three Properties Of A Base The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Aqueous solutions of bases are also electrolytes. Aqueous solutions of bases are also electrolytes. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons. What Is The Three Properties Of A Base.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free What Is The Three Properties Of A Base The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity. Bases have properties that mostly contrast with those of acids. Bases have properties that mostly contrast with those of acids. Common bases include metal oxides, hydroxides or carbonates. Aqueous solutions of bases are also electrolytes. Bases can be either. What Is The Three Properties Of A Base.