Low Ionic Strength Buffer Example . Determines the buffer ion concentrations but. Use this formula to calculate ionic strength: These commonly observed problems can be. Let “i” equal ionic strength of the solution. Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Learn how to overcome the challenges of measuring ph in purified water and other low conductivity liquids, such as. The formula in step 1. The molar strength of the two components of the buffer should be chosen to give adequate buffering. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. See examples, equations, and tips for making and using buffers in. Buffer capacity and ionic strength: Ionic strength can be molar (mol/l solution) or molal (mol/kg. One of the main characteristics of a solution with dissolved ions is the ionic strength.
from www.pinterest.es
Use this formula to calculate ionic strength: Determines the buffer ion concentrations but. See examples, equations, and tips for making and using buffers in. These commonly observed problems can be. One of the main characteristics of a solution with dissolved ions is the ionic strength. Let “i” equal ionic strength of the solution. Learn how to overcome the challenges of measuring ph in purified water and other low conductivity liquids, such as. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. The formula in step 1. Ionic strength can be molar (mol/l solution) or molal (mol/kg.
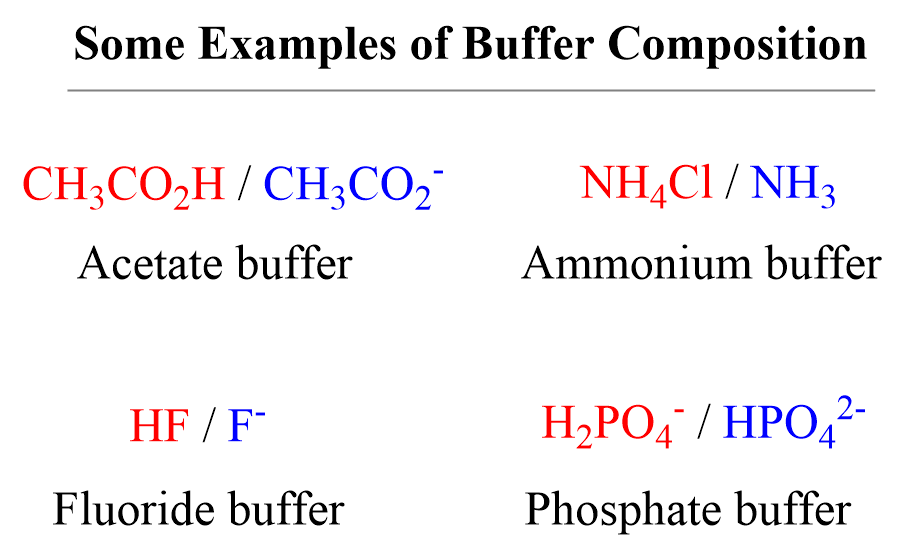
Understanding Buffer Solution Chemistry A Complete Guide
Low Ionic Strength Buffer Example These commonly observed problems can be. These commonly observed problems can be. Determines the buffer ion concentrations but. The molar strength of the two components of the buffer should be chosen to give adequate buffering. The formula in step 1. Use this formula to calculate ionic strength: Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. Learn how to overcome the challenges of measuring ph in purified water and other low conductivity liquids, such as. See examples, equations, and tips for making and using buffers in. Buffer capacity and ionic strength: Let “i” equal ionic strength of the solution. One of the main characteristics of a solution with dissolved ions is the ionic strength. Ionic strength can be molar (mol/l solution) or molal (mol/kg. Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Low Ionic Strength Buffer Example Ionic strength can be molar (mol/l solution) or molal (mol/kg. One of the main characteristics of a solution with dissolved ions is the ionic strength. Buffer capacity and ionic strength: Use this formula to calculate ionic strength: These commonly observed problems can be. Determines the buffer ion concentrations but. Learn how to overcome the challenges of measuring ph in purified. Low Ionic Strength Buffer Example.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Low Ionic Strength Buffer Example The formula in step 1. Ionic strength can be molar (mol/l solution) or molal (mol/kg. Determines the buffer ion concentrations but. Buffer capacity and ionic strength: The molar strength of the two components of the buffer should be chosen to give adequate buffering. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the. Low Ionic Strength Buffer Example.
From www.researchgate.net
Effect of ionic strength of the acetate buffer at pH 4.0 and phosphate Low Ionic Strength Buffer Example Ionic strength can be molar (mol/l solution) or molal (mol/kg. Use this formula to calculate ionic strength: Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. See examples, equations, and tips for making and using buffers in. The molar strength of the two components of the buffer. Low Ionic Strength Buffer Example.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Low Ionic Strength Buffer Example These commonly observed problems can be. One of the main characteristics of a solution with dissolved ions is the ionic strength. Let “i” equal ionic strength of the solution. Ionic strength can be molar (mol/l solution) or molal (mol/kg. See examples, equations, and tips for making and using buffers in. Starting with a given ph, the buffer ion concentrations can. Low Ionic Strength Buffer Example.
From www.researchgate.net
The effect of the ionic strength of a mixture of various buffers on the Low Ionic Strength Buffer Example Use this formula to calculate ionic strength: These commonly observed problems can be. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. Ionic strength can be molar (mol/l solution) or molal (mol/kg. See examples, equations, and tips for making and using buffers in. The molar strength of. Low Ionic Strength Buffer Example.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Low Ionic Strength Buffer Example Determines the buffer ion concentrations but. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Buffer capacity and ionic strength: One of the main characteristics of a solution with. Low Ionic Strength Buffer Example.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Low Ionic Strength Buffer Example The formula in step 1. See examples, equations, and tips for making and using buffers in. Buffer capacity and ionic strength: Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. Let “i” equal ionic strength of the solution. One of the main characteristics of a solution with. Low Ionic Strength Buffer Example.
From www.researchgate.net
Activity of T4 lysozyme in low ionic strength buffer. M. luteus Low Ionic Strength Buffer Example See examples, equations, and tips for making and using buffers in. Ionic strength can be molar (mol/l solution) or molal (mol/kg. Determines the buffer ion concentrations but. The molar strength of the two components of the buffer should be chosen to give adequate buffering. Let “i” equal ionic strength of the solution. The formula in step 1. One of the. Low Ionic Strength Buffer Example.
From www.reagents.com
Low Level TISAB II Ionic Strength Adjustment Buffer, 500 mL Reagents Low Ionic Strength Buffer Example Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. Use this formula to calculate ionic strength: Let “i” equal ionic strength of the solution. Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Determines the buffer ion concentrations. Low Ionic Strength Buffer Example.
From www.revvity.com
HTRF PPI Low Ionic Strength Europium Detection buffer, 220 mL Low Ionic Strength Buffer Example One of the main characteristics of a solution with dissolved ions is the ionic strength. Use this formula to calculate ionic strength: See examples, equations, and tips for making and using buffers in. Let “i” equal ionic strength of the solution. Determines the buffer ion concentrations but. The formula in step 1. The molar strength of the two components of. Low Ionic Strength Buffer Example.
From www.researchgate.net
(a) Effect of pH and (b) ionic strength of selected buffer on the Low Ionic Strength Buffer Example Learn how to overcome the challenges of measuring ph in purified water and other low conductivity liquids, such as. Ionic strength can be molar (mol/l solution) or molal (mol/kg. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. These commonly observed problems can be. Use this formula. Low Ionic Strength Buffer Example.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Low Ionic Strength Buffer Example Buffer capacity and ionic strength: Determines the buffer ion concentrations but. These commonly observed problems can be. Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Learn how to overcome the challenges of measuring ph in purified water and other low conductivity liquids, such as. Ionic strength can be molar (mol/l. Low Ionic Strength Buffer Example.
From www.researchgate.net
3 Comparison of BPDNA condensates at low and high ionic strength Low Ionic Strength Buffer Example The formula in step 1. Let “i” equal ionic strength of the solution. The molar strength of the two components of the buffer should be chosen to give adequate buffering. See examples, equations, and tips for making and using buffers in. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength. Low Ionic Strength Buffer Example.
From www.slideserve.com
PPT Sensitization and Agglutination PowerPoint Presentation ID3923883 Low Ionic Strength Buffer Example One of the main characteristics of a solution with dissolved ions is the ionic strength. Let “i” equal ionic strength of the solution. These commonly observed problems can be. See examples, equations, and tips for making and using buffers in. Ionic strength can be molar (mol/l solution) or molal (mol/kg. Learn how to calculate the ionic strength of buffers, especially. Low Ionic Strength Buffer Example.
From www.slideserve.com
PPT Hemoglobin Electrophoresis PowerPoint Presentation, free download Low Ionic Strength Buffer Example Ionic strength can be molar (mol/l solution) or molal (mol/kg. Use this formula to calculate ionic strength: Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Determines the buffer ion concentrations but. The formula in step 1. One of the main characteristics of a solution with dissolved ions is the ionic. Low Ionic Strength Buffer Example.
From www.researchgate.net
Low ionic strength buffers supporting specific hybridization. Using the Low Ionic Strength Buffer Example Ionic strength can be molar (mol/l solution) or molal (mol/kg. Learn how to overcome the challenges of measuring ph in purified water and other low conductivity liquids, such as. See examples, equations, and tips for making and using buffers in. Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Let “i”. Low Ionic Strength Buffer Example.
From www.researchgate.net
Effect of ionic strength on the chemical relaxation time C at pH 5.5 Low Ionic Strength Buffer Example One of the main characteristics of a solution with dissolved ions is the ionic strength. Let “i” equal ionic strength of the solution. Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. The formula in step 1. Starting with a given ph, the buffer ion concentrations can be calculated including temperature. Low Ionic Strength Buffer Example.
From www.researchgate.net
Calibration curve of the ISFET in drops of buffer with low ionic Low Ionic Strength Buffer Example Buffer capacity and ionic strength: The molar strength of the two components of the buffer should be chosen to give adequate buffering. Use this formula to calculate ionic strength: See examples, equations, and tips for making and using buffers in. Determines the buffer ion concentrations but. The formula in step 1. Learn how to calculate the ionic strength of buffers,. Low Ionic Strength Buffer Example.
From www.youtube.com
Ionic Equilibria V Identifying Buffer Solutions YouTube Low Ionic Strength Buffer Example Use this formula to calculate ionic strength: Buffer capacity and ionic strength: Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. Let “i” equal ionic strength of the solution. Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph.. Low Ionic Strength Buffer Example.
From www.researchgate.net
8 A temporal change in morphology of DNA condensates is not correlated Low Ionic Strength Buffer Example Let “i” equal ionic strength of the solution. One of the main characteristics of a solution with dissolved ions is the ionic strength. Determines the buffer ion concentrations but. Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Starting with a given ph, the buffer ion concentrations can be calculated including. Low Ionic Strength Buffer Example.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Low Ionic Strength Buffer Example Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Ionic strength can be molar (mol/l solution) or molal (mol/kg. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. See examples, equations, and tips for making and using buffers. Low Ionic Strength Buffer Example.
From www.coleparmer.com
Spex CertiPrep ISBUF3500 Low Level TISAB II Ionic Strength Adjustment Low Ionic Strength Buffer Example These commonly observed problems can be. Buffer capacity and ionic strength: Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Determines the buffer ion concentrations but. See examples, equations, and tips for making and using buffers in. Learn how to overcome the challenges of measuring ph in purified water and other. Low Ionic Strength Buffer Example.
From www.researchgate.net
A schematic of a calcite surface in low and high ionic strength Low Ionic Strength Buffer Example Let “i” equal ionic strength of the solution. The molar strength of the two components of the buffer should be chosen to give adequate buffering. One of the main characteristics of a solution with dissolved ions is the ionic strength. See examples, equations, and tips for making and using buffers in. Ionic strength can be molar (mol/l solution) or molal. Low Ionic Strength Buffer Example.
From www.researchgate.net
Compositions of Low Ionic Strength Reference Waters Used in Download Low Ionic Strength Buffer Example The formula in step 1. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. Let “i” equal ionic strength of the solution. See examples, equations, and tips for making and using buffers in. Learn how to overcome the challenges of measuring ph in purified water and other. Low Ionic Strength Buffer Example.
From www.camlab.co.uk
Low Ionic Strength Buffers pH Values At 20°C Low Ionic Strength Buffer Example The formula in step 1. Use this formula to calculate ionic strength: Buffer capacity and ionic strength: These commonly observed problems can be. Ionic strength can be molar (mol/l solution) or molal (mol/kg. Determines the buffer ion concentrations but. See examples, equations, and tips for making and using buffers in. The molar strength of the two components of the buffer. Low Ionic Strength Buffer Example.
From www.researchgate.net
Low ionic strength experiments in water presaturated with calcite and Low Ionic Strength Buffer Example Buffer capacity and ionic strength: Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. Determines the buffer ion concentrations but. See examples, equations, and tips for making and using. Low Ionic Strength Buffer Example.
From www.vacutaineradditives.com
Low Ionic Strength Hygroscopic Tromethamine Tris Buffer Low Ionic Strength Buffer Example Determines the buffer ion concentrations but. Use this formula to calculate ionic strength: One of the main characteristics of a solution with dissolved ions is the ionic strength. Ionic strength can be molar (mol/l solution) or molal (mol/kg. Buffer capacity and ionic strength: Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the. Low Ionic Strength Buffer Example.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Low Ionic Strength Buffer Example Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Let “i” equal ionic strength of the solution. Learn how to overcome the challenges of measuring ph in purified water and other low conductivity liquids, such as. These commonly observed problems can be. One of the main characteristics of a solution with. Low Ionic Strength Buffer Example.
From www.researchgate.net
Calibration curve of the ISFET in drops of buffer with low ionic Low Ionic Strength Buffer Example The molar strength of the two components of the buffer should be chosen to give adequate buffering. Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Learn how to overcome the challenges of measuring ph in purified water and other low conductivity liquids, such as. Let “i” equal ionic strength of. Low Ionic Strength Buffer Example.
From answers.seneye.com
Effect of ionic strength on PH tests Seneye Low Ionic Strength Buffer Example One of the main characteristics of a solution with dissolved ions is the ionic strength. These commonly observed problems can be. Buffer capacity and ionic strength: Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. Use this formula to calculate ionic strength: The formula in step 1.. Low Ionic Strength Buffer Example.
From www.slideserve.com
PPT Hemoglobin Electrophoresis PowerPoint Presentation, free download Low Ionic Strength Buffer Example Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Determines the buffer ion concentrations but. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. One of the main characteristics of a solution with dissolved ions is the ionic. Low Ionic Strength Buffer Example.
From www.researchgate.net
Scheme of the ion's dissociation and hydration in buffer solution with Low Ionic Strength Buffer Example See examples, equations, and tips for making and using buffers in. One of the main characteristics of a solution with dissolved ions is the ionic strength. The molar strength of the two components of the buffer should be chosen to give adequate buffering. Learn how to overcome the challenges of measuring ph in purified water and other low conductivity liquids,. Low Ionic Strength Buffer Example.
From www.researchgate.net
Fig. S5 Ionic Strength of the buffer (left axis) and added NaCl Low Ionic Strength Buffer Example The formula in step 1. The molar strength of the two components of the buffer should be chosen to give adequate buffering. Let “i” equal ionic strength of the solution. Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. One of the main characteristics of a solution with dissolved ions is. Low Ionic Strength Buffer Example.
From slidetodoc.com
Lab 10 Electrophoresis Analytical biochemistry lab KAUbiochemistry dep Low Ionic Strength Buffer Example Learn how to calculate the ionic strength of buffers, especially pipes, which is divalent and depends on ph. Buffer capacity and ionic strength: Let “i” equal ionic strength of the solution. One of the main characteristics of a solution with dissolved ions is the ionic strength. Learn how to overcome the challenges of measuring ph in purified water and other. Low Ionic Strength Buffer Example.
From cymitquimica.com
Reagecon pH 4.10 Low Ionic Strength Buffer Solution at 20°C 07LS41 Low Ionic Strength Buffer Example Let “i” equal ionic strength of the solution. Use this formula to calculate ionic strength: See examples, equations, and tips for making and using buffers in. One of the main characteristics of a solution with dissolved ions is the ionic strength. Buffer capacity and ionic strength: Ionic strength can be molar (mol/l solution) or molal (mol/kg. Learn how to calculate. Low Ionic Strength Buffer Example.