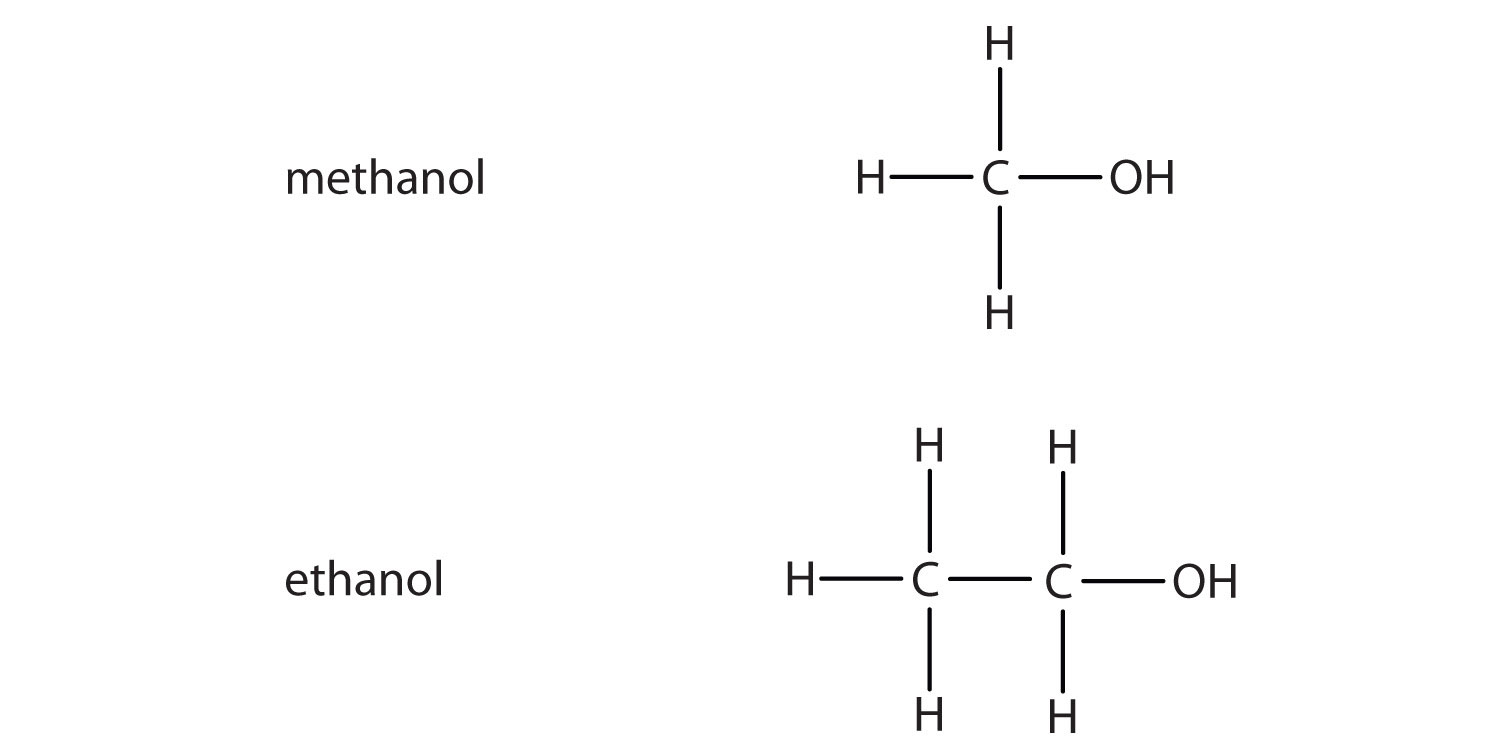
What Is The Molecular Structure Of Alcohol . Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Discuss the factors that are believed to determine the. Alcohols may be considered as organic derivatives of water. A secondary (2°) alcohol is one. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). It examines in some detail. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Ethanol, ch 3 ch 2 oh, also. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Its general formula is rch 2 oh.
from saylordotorg.github.io
Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. It examines in some detail. Alcohols may be considered as organic derivatives of water. Ethanol, ch 3 ch 2 oh, also. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. A secondary (2°) alcohol is one. Discuss the factors that are believed to determine the. Its general formula is rch 2 oh. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass.
Covalent Bonding and Simple Molecular Compounds
What Is The Molecular Structure Of Alcohol Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Discuss the factors that are believed to determine the. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Alcohols may be considered as organic derivatives of water. A secondary (2°) alcohol is one. It examines in some detail. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). Ethanol, ch 3 ch 2 oh, also. Its general formula is rch 2 oh. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass.
From www.sciencerifi.co.uk
Alkanes, alkenes, Organic chemistry What Is The Molecular Structure Of Alcohol Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. A secondary (2°) alcohol is one. Discuss the factors that are believed to determine the. Alcohols may be considered as organic derivatives of water. A primary (1°) alcohol is one in which the carbon atom (in red). What Is The Molecular Structure Of Alcohol.
From www.alamy.com
Alcohol (ethanol, ethyl alcohol) molecule, chemical structure. Skeletal What Is The Molecular Structure Of Alcohol A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. A secondary (2°) alcohol is one. Discuss the factors that are believed. What Is The Molecular Structure Of Alcohol.
From mavink.com
Estructura Del Alcohol What Is The Molecular Structure Of Alcohol Its general formula is rch 2 oh. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Alcohols may be considered as organic derivatives of water. It examines in some detail. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group. What Is The Molecular Structure Of Alcohol.
From winespeed.com
alcoholmolecule WineSpeed What Is The Molecular Structure Of Alcohol Ethanol, ch 3 ch 2 oh, also. It examines in some detail. Discuss the factors that are believed to determine the. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group. What Is The Molecular Structure Of Alcohol.
From mavink.com
Alcohol Skeletal Structure What Is The Molecular Structure Of Alcohol A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. A secondary (2°) alcohol is one. It examines in some detail. Discuss the factors that are believed to determine. What Is The Molecular Structure Of Alcohol.
From stock.adobe.com
Alcohol (ethanol, ethyl alcohol) molecule, chemical structure. Skeletal What Is The Molecular Structure Of Alcohol Ethanol, ch 3 ch 2 oh, also. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. A secondary (2°) alcohol is one. Its general formula is rch 2 oh. Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon. What Is The Molecular Structure Of Alcohol.
From www.zazzle.com
alcohol_chemical_formula_postcardre03cbdb2950740f3ac80852c782fe301 What Is The Molecular Structure Of Alcohol Its general formula is rch 2 oh. Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Ethanol, ch 3 ch 2 oh, also. Alcohols may be considered as organic derivatives of water. This page defines an alcohol, and explains the differences between primary, secondary and tertiary. What Is The Molecular Structure Of Alcohol.
From stock.adobe.com
Vector illustration of the molecular structural formula of alcohol with What Is The Molecular Structure Of Alcohol A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). Alcohols may be considered as organic derivatives of water. It examines in some detail. Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of. What Is The Molecular Structure Of Alcohol.
From www.thoughtco.com
Absolute Alcohol Definition and Formula What Is The Molecular Structure Of Alcohol Ethanol, ch 3 ch 2 oh, also. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. It examines in some detail. Alcohols may be considered as organic derivatives of water. Discuss the. What Is The Molecular Structure Of Alcohol.
From www.alamy.com
Ethanol Alcohol Molecular structure vector skeletal formula Stock What Is The Molecular Structure Of Alcohol Alcohols may be considered as organic derivatives of water. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Discuss the factors. What Is The Molecular Structure Of Alcohol.
From manashsubhaditya.blogspot.com
Manash (Subhaditya Edusoft) Organic Chemistry Part 2 Alcohols What Is The Molecular Structure Of Alcohol Its general formula is rch 2 oh. Alcohols may be considered as organic derivatives of water. Ethanol, ch 3 ch 2 oh, also. Discuss the factors that are believed to determine the. It examines in some detail. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. A. What Is The Molecular Structure Of Alcohol.
From www.vecteezy.com
Chemistry model molecule ethanol C2H5OH scientific element formula What Is The Molecular Structure Of Alcohol Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. It examines in some detail. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). This page defines an alcohol, and explains. What Is The Molecular Structure Of Alcohol.
From www.alamy.com
Alcohol (ethanol, ethyl alcohol) molecule, chemical structure. Skeletal What Is The Molecular Structure Of Alcohol Its general formula is rch 2 oh. A secondary (2°) alcohol is one. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). Discuss the factors that are believed. What Is The Molecular Structure Of Alcohol.
From courses.lumenlearning.com
14.2 Alcohols Nomenclature and Classification The Basics of General What Is The Molecular Structure Of Alcohol Discuss the factors that are believed to determine the. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Alcohol, any of a class of organic compounds with one or more hydroxyl groups. What Is The Molecular Structure Of Alcohol.
From understandingstem.com
Alcohols names and formulas chemistry) — Understanding STEM What Is The Molecular Structure Of Alcohol Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Discuss the factors that are believed to determine the. Alcohols may be considered as organic derivatives of water. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached. What Is The Molecular Structure Of Alcohol.
From www.dreamstime.com
Simple Alcoholic Compounds, Molecular Models and Formulas Stock Vector What Is The Molecular Structure Of Alcohol Ethanol, ch 3 ch 2 oh, also. Discuss the factors that are believed to determine the. A secondary (2°) alcohol is one. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. It examines in some detail. Alcohols may be considered as organic derivatives of water. Alcohol, any of a class of organic compounds with. What Is The Molecular Structure Of Alcohol.
From ck12.org
Functional Groups CK12 Foundation What Is The Molecular Structure Of Alcohol Ethanol, ch 3 ch 2 oh, also. Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Explain why the boiling points of alcohols and phenols are much higher than those of. What Is The Molecular Structure Of Alcohol.
From mavink.com
Estructura Del Alcohol What Is The Molecular Structure Of Alcohol It examines in some detail. Discuss the factors that are believed to determine the. Alcohols may be considered as organic derivatives of water. Its general formula is rch 2 oh. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Explain why the boiling points of alcohols and phenols are much higher than those of. What Is The Molecular Structure Of Alcohol.
From www.alamy.com
Ethanol (alcohol) molecule, chemical structure. Atoms are represented What Is The Molecular Structure Of Alcohol Alcohols may be considered as organic derivatives of water. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. A primary (1°) alcohol is one in which the carbon atom (in red) with. What Is The Molecular Structure Of Alcohol.
From www.dreamstime.com
Structural Chemical Formula. Ethanol. C2H5OH. 3d Rendering. Digital What Is The Molecular Structure Of Alcohol It examines in some detail. Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. A secondary (2°) alcohol is one. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Ethanol, ch 3 ch 2 oh, also. Discuss the factors that. What Is The Molecular Structure Of Alcohol.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Alcohols What Is The Molecular Structure Of Alcohol A secondary (2°) alcohol is one. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Its general formula is rch 2 oh. It examines in some detail. Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Ethanol, ch 3 ch. What Is The Molecular Structure Of Alcohol.
From www.dreamstime.com
Ethanol, Ethyl Alcohol Molecule, Chemical Structure. Skeletal Formula What Is The Molecular Structure Of Alcohol This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Alcohols may be considered as organic derivatives of water. Ethanol, ch 3 ch 2 oh, also. Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Explain why the boiling points of. What Is The Molecular Structure Of Alcohol.
From www.alamy.com
3d render of molecular structure of alcohol isolated over white What Is The Molecular Structure Of Alcohol It examines in some detail. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). A secondary (2°) alcohol is one. Discuss the factors that are believed to determine the. Its general formula is rch 2 oh. Explain why the boiling points of alcohols. What Is The Molecular Structure Of Alcohol.
From ar.inspiredpencil.com
Alcohol Structural Formula What Is The Molecular Structure Of Alcohol A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). Discuss the factors that are believed to determine the. Its general formula is rch 2 oh. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc.,. What Is The Molecular Structure Of Alcohol.
From saylordotorg.github.io
Covalent Bonding and Simple Molecular Compounds What Is The Molecular Structure Of Alcohol Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Ethanol, ch 3 ch 2 oh, also. It examines in some detail. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in. What Is The Molecular Structure Of Alcohol.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Primary alcohol (1o alcohol What Is The Molecular Structure Of Alcohol Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Alcohols may be considered as organic derivatives of water. A secondary (2°) alcohol is one. Alcohol, any of a class of organic compounds. What Is The Molecular Structure Of Alcohol.
From janelaurachemistry.weebly.com
ALCOHOL organic Chemistry What Is The Molecular Structure Of Alcohol Discuss the factors that are believed to determine the. A secondary (2°) alcohol is one. Its general formula is rch 2 oh. Ethanol, ch 3 ch 2 oh, also. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). Explain why the boiling points. What Is The Molecular Structure Of Alcohol.
From www.adda247.com
Ethanol Formula Ethyl Alcohol Formula, Structure What Is The Molecular Structure Of Alcohol A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). It examines in some detail. Its general formula is rch 2 oh. A secondary (2°) alcohol is one. Ethanol, ch 3 ch 2 oh, also. Alcohol, any of a class of organic compounds with. What Is The Molecular Structure Of Alcohol.
From www.alamy.com
Ethanol, C2H5OH molecule. It is a primary alcohol, an alkyl alcohol What Is The Molecular Structure Of Alcohol A secondary (2°) alcohol is one. It examines in some detail. Discuss the factors that are believed to determine the. Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers,. What Is The Molecular Structure Of Alcohol.
From techiescientist.com
Is Ethanol (C2H5OH) Polar or Nonpolar? Techiescientist What Is The Molecular Structure Of Alcohol Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Ethanol, ch 3 ch 2 oh, also. Alcohols may be considered as organic derivatives of water. A secondary (2°) alcohol is one. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh. What Is The Molecular Structure Of Alcohol.
From www.alamy.com
Alcohol (ethanol, ethyl alcohol) molecule, chemical structure. Stylized What Is The Molecular Structure Of Alcohol A secondary (2°) alcohol is one. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Its general formula is rch 2 oh. Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Discuss the. What Is The Molecular Structure Of Alcohol.
From www.alamy.com
3d render of molecular structure of alcohol isolated over white What Is The Molecular Structure Of Alcohol It examines in some detail. A secondary (2°) alcohol is one. Ethanol, ch 3 ch 2 oh, also. Alcohols may be considered as organic derivatives of water. Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. This page defines an alcohol, and explains the differences between. What Is The Molecular Structure Of Alcohol.
From www.shutterstock.com
Ethanol (Alcohol) Molecule, Chemical Structure. Three Representations What Is The Molecular Structure Of Alcohol It examines in some detail. Discuss the factors that are believed to determine the. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Ethanol, ch 3 ch 2 oh, also. Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Its. What Is The Molecular Structure Of Alcohol.
From www.dreamstime.com
Diagram Molecule of Ethanol Stock Vector Illustration of concept What Is The Molecular Structure Of Alcohol Alcohol, any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Explain why the boiling points of alcohols and phenols are much higher than those of alkanes, ethers, etc., of similar molecular mass. Discuss the factors that are believed to determine the. Alcohols may be considered as organic. What Is The Molecular Structure Of Alcohol.
From thechemistrynotes.com
Alcohols Structure, Classification, Preparation, Properties, Uses What Is The Molecular Structure Of Alcohol This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Alcohols may be considered as organic derivatives of water. A secondary (2°) alcohol is one. A primary (1°) alcohol is one in which the carbon atom (in red) with the oh group is attached to one other carbon atom (in blue). Explain why the boiling. What Is The Molecular Structure Of Alcohol.