Cap Requirements For Quality Control . january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. Our unique, reciprocal, peer inspection model benefits. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. where can i find the cap checklist requirements for iqcp? adopting these guidelines helps pathologists and laboratory professionals to provide more effective testing with consistent, high. Cap inspections are educational, not punitive. the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. Five specific iqcp requirements are included. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into.
from www.westgard.com
adopting these guidelines helps pathologists and laboratory professionals to provide more effective testing with consistent, high. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. where can i find the cap checklist requirements for iqcp? Our unique, reciprocal, peer inspection model benefits. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. Cap inspections are educational, not punitive. Five specific iqcp requirements are included.
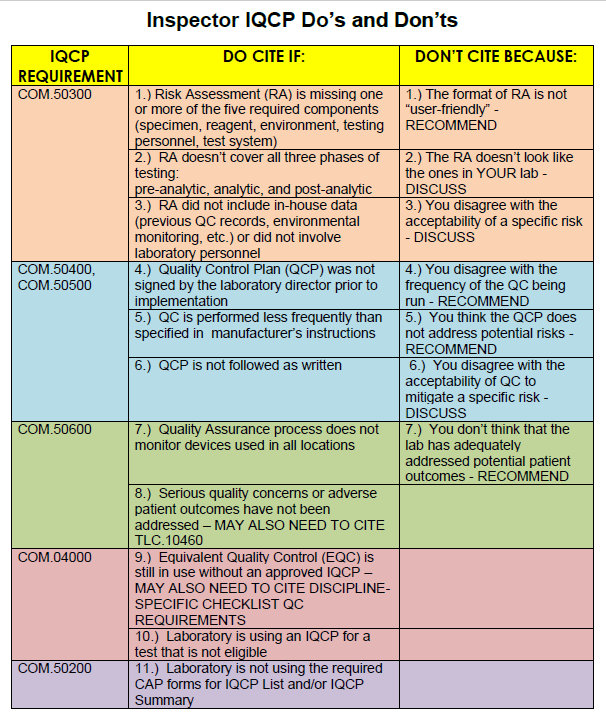
Your RiskFree IQCP Inspection Tips Westgard
Cap Requirements For Quality Control Cap inspections are educational, not punitive. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. adopting these guidelines helps pathologists and laboratory professionals to provide more effective testing with consistent, high. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. Five specific iqcp requirements are included. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. where can i find the cap checklist requirements for iqcp? Cap inspections are educational, not punitive. the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. Our unique, reciprocal, peer inspection model benefits.
From www.researchgate.net
Relationship between customer requirements and technical descriptors Cap Requirements For Quality Control the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. Five specific iqcp requirements are included. participants of the cap accreditation programs may download the checklists from the cap website (cap.org). Cap Requirements For Quality Control.
From www.aruplab.com
ARUP Announces Successful Completion of CAP ISO 15189 Assessment ARUP Cap Requirements For Quality Control adopting these guidelines helps pathologists and laboratory professionals to provide more effective testing with consistent, high. january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. the quality control requirements for these. Cap Requirements For Quality Control.
From old.sermitsiaq.ag
Llc Cap Table Template Cap Requirements For Quality Control january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. where can i find the cap checklist requirements for iqcp? Our unique, reciprocal, peer inspection model benefits. Five specific iqcp requirements are included. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by. Cap Requirements For Quality Control.
From studylib.net
Individualized Quality Control Plan Summary Cap Requirements For Quality Control Five specific iqcp requirements are included. january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging. Cap Requirements For Quality Control.
From arelibilpennington.blogspot.com
Cap All Common Checklist ArelibilPennington Cap Requirements For Quality Control the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. Cap inspections are educational, not punitive. Five specific iqcp requirements are included. participants of the cap accreditation programs may download the. Cap Requirements For Quality Control.
From www.slideserve.com
PPT STANEVAL PowerPoint Presentation, free download ID394374 Cap Requirements For Quality Control the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. Our unique, reciprocal, peer inspection model benefits. where can i find the cap checklist requirements for iqcp? participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. adopting these guidelines. Cap Requirements For Quality Control.
From www.slideserve.com
PPT Understanding Nonprofits and the National Forest Foundation (NFF Cap Requirements For Quality Control Cap inspections are educational, not punitive. january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. where can i find the cap checklist requirements for iqcp? the cap's quality management. Cap Requirements For Quality Control.
From www.projectmanager.com
Capacity Planning Strategies, Benefits and Best Practices Cap Requirements For Quality Control the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. adopting these guidelines helps pathologists and laboratory professionals to provide more effective testing with consistent, high. Five specific iqcp requirements are included. Our unique, reciprocal, peer inspection model benefits. where can i find the cap checklist requirements for iqcp?. Cap Requirements For Quality Control.
From innopharmatechservices.com
Quality Control Analysts Innopharma Technology Services Cap Requirements For Quality Control participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. Five specific iqcp requirements are included. where can i find the cap checklist requirements for iqcp? january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. the cap's quality management. Cap Requirements For Quality Control.
From www.scribd.com
Corrective Action Plan (CAP) Template Systems Science Engineering Cap Requirements For Quality Control participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. Our unique, reciprocal, peer inspection model benefits. the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. participants of the cap accreditation programs may download the checklists from the cap website. Cap Requirements For Quality Control.
From xpert.helpscoutdocs.com
List of Updated CAP Checklists 2019 xPert/eFRM Cap Requirements For Quality Control the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. where can i find the cap checklist requirements for iqcp? Five specific iqcp requirements are included. Our unique, reciprocal, peer inspection model benefits.. Cap Requirements For Quality Control.
From www.westgard.com
Your RiskFree IQCP Inspection Tips Westgard Cap Requirements For Quality Control adopting these guidelines helps pathologists and laboratory professionals to provide more effective testing with consistent, high. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. Our unique, reciprocal, peer inspection model benefits. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by. Cap Requirements For Quality Control.
From www.slideserve.com
PPT Condition Assessment Programme (CAP) PowerPoint Presentation Cap Requirements For Quality Control the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. Our unique, reciprocal, peer inspection model benefits. where can i find the cap checklist requirements for iqcp? participants of the cap accreditation. Cap Requirements For Quality Control.
From www.chemistry.ucla.edu
Personal Protective Equipment UCLA Cap Requirements For Quality Control january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. Cap inspections are educational, not punitive. where can i find the cap checklist requirements for iqcp? Five specific iqcp requirements are included. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. . Cap Requirements For Quality Control.
From www.slideshare.net
CAP guidelines 2010 Cap Requirements For Quality Control where can i find the cap checklist requirements for iqcp? Our unique, reciprocal, peer inspection model benefits. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. adopting these guidelines helps pathologists and laboratory professionals to provide more effective testing with consistent, high. Cap inspections are educational, not punitive.. Cap Requirements For Quality Control.
From studylib.net
CAPS Grading Scale Cap Requirements For Quality Control where can i find the cap checklist requirements for iqcp? adopting these guidelines helps pathologists and laboratory professionals to provide more effective testing with consistent, high. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. the quality control requirements for these methods are defined in the method specific sections. Cap Requirements For Quality Control.
From www.vectorstock.com
Quality control approved icon Royalty Free Vector Image Cap Requirements For Quality Control the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. where can i find the cap checklist requirements for iqcp? january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. Cap inspections are educational, not punitive. Our unique, reciprocal, peer inspection model benefits.. Cap Requirements For Quality Control.
From pdfslide.net
Common IPC6012D/DS Requirement Differences Per Class 2 Class 3 Class Cap Requirements For Quality Control Our unique, reciprocal, peer inspection model benefits. where can i find the cap checklist requirements for iqcp? the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. january 1, 2016,. Cap Requirements For Quality Control.
From soby.world.edu
Maximizing Safety Essential Guidelines for Hard Hat Impact Protection Cap Requirements For Quality Control participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. where can i find the cap checklist requirements for iqcp? participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. adopting these guidelines helps pathologists and laboratory professionals to provide. Cap Requirements For Quality Control.
From nationalgriefawarenessday.com
Corrective Action Plan Template Business Cap Requirements For Quality Control where can i find the cap checklist requirements for iqcp? participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. the quality control requirements for these methods are defined in the method. Cap Requirements For Quality Control.
From www.examples.com
Corrective Action Plan 11+ Examples, Format, Doc, Word, Elements Cap Requirements For Quality Control where can i find the cap checklist requirements for iqcp? the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. Our unique, reciprocal, peer inspection model benefits. Five specific iqcp requirements are included. Cap inspections are educational, not punitive. the quality control requirements for these methods are defined in the method. Cap Requirements For Quality Control.
From slidetodoc.com
CAP Accreditation and Checklists Update California Clinical Laboratory Cap Requirements For Quality Control the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. Cap inspections are educational, not punitive. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. where can i find the cap checklist requirements for iqcp? adopting these guidelines helps pathologists and. Cap Requirements For Quality Control.
From www.slideshare.net
caps document Cap Requirements For Quality Control Cap inspections are educational, not punitive. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. adopting these guidelines helps pathologists and laboratory professionals to provide more effective testing with consistent, high. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. Five. Cap Requirements For Quality Control.
From exopzfqjk.blob.core.windows.net
Cap Project Requirements at Charles Fairbanks blog Cap Requirements For Quality Control participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. Five specific iqcp requirements are. Cap Requirements For Quality Control.
From www.slideserve.com
PPT Point of Care Competency Testing at Lancaster General Hospital Cap Requirements For Quality Control Five specific iqcp requirements are included. where can i find the cap checklist requirements for iqcp? Cap inspections are educational, not punitive. the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. Our unique, reciprocal, peer inspection model benefits. participants of the cap accreditation programs may download the checklists. Cap Requirements For Quality Control.
From caps123.co.za
Top tips and guidelines for CAPS Assessment CAPS 123 Cap Requirements For Quality Control Five specific iqcp requirements are included. Our unique, reciprocal, peer inspection model benefits. january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. adopting these guidelines helps pathologists and laboratory professionals. Cap Requirements For Quality Control.
From www.slideserve.com
PPT E pluribus unum Regulatory Perspectives on Validating Moderately Cap Requirements For Quality Control the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. Our unique, reciprocal, peer inspection model benefits. where can i find the cap checklist requirements for iqcp? january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. the quality control requirements for. Cap Requirements For Quality Control.
From business901.com
Description of Check Act Plan Do Model Cap Requirements For Quality Control Our unique, reciprocal, peer inspection model benefits. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. Cap inspections are educational, not punitive. the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. Five specific iqcp requirements are included. participants of. Cap Requirements For Quality Control.
From www.researchgate.net
IDSA / ATS criteria of severe CAP Download Scientific Diagram Cap Requirements For Quality Control Cap inspections are educational, not punitive. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. Our unique, reciprocal, peer inspection model benefits. january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. Five specific iqcp requirements are included. the quality. Cap Requirements For Quality Control.
From www.templateroller.com
CAP Form 71G Fill Out, Sign Online and Download Printable PDF Cap Requirements For Quality Control Cap inspections are educational, not punitive. adopting these guidelines helps pathologists and laboratory professionals to provide more effective testing with consistent, high. Our unique, reciprocal, peer inspection model benefits. where can i find the cap checklist requirements for iqcp? participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into.. Cap Requirements For Quality Control.
From fortaps.com
What are the quality checks that an end cap has to pass? Cap Requirements For Quality Control Cap inspections are educational, not punitive. Five specific iqcp requirements are included. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. where can i find the cap checklist requirements for iqcp? participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging. Cap Requirements For Quality Control.
From www.antaresvisiongroup.com
Tethered Caps New Solutions For Effective Inline Quality Control Cap Requirements For Quality Control adopting these guidelines helps pathologists and laboratory professionals to provide more effective testing with consistent, high. Five specific iqcp requirements are included. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging. Cap Requirements For Quality Control.
From www.slideshare.net
caps document Cap Requirements For Quality Control participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. participants of the cap accreditation programs may download the checklists from the cap website (cap.org) by logging into. january 1,. Cap Requirements For Quality Control.
From studylib.net
CAP Aircraft Inspection Checklist Inspection Item (Installed Cap Requirements For Quality Control Five specific iqcp requirements are included. adopting these guidelines helps pathologists and laboratory professionals to provide more effective testing with consistent, high. january 1, 2016, all laboratories performing nonwaived testing must follow the minimum cap requirements and default clia quality. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. . Cap Requirements For Quality Control.
From www.youtube.com
CAP Accreditation 10 Steps on the Path to Laboratory Excellence YouTube Cap Requirements For Quality Control the quality control requirements for these methods are defined in the method specific sections of the applicable checklists. the cap's quality management tools strengthen your knowledge of key laboratory processes and provides information for. where can i find the cap checklist requirements for iqcp? adopting these guidelines helps pathologists and laboratory professionals to provide more effective. Cap Requirements For Quality Control.